Abstract
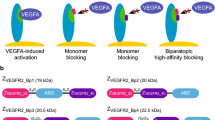
Human tear lipocalin (Tlc) was utilized as a protein scaffold to engineer an Anticalin that specifically binds and functionally blocks vascular endothelial growth factor A (VEGF-A), a pivotal inducer of physiological angiogenesis that also plays a crucial role in several neovascular diseases. Starting from a naive combinatorial library where residues that form the natural ligand-binding site of Tlc were randomized, followed by affinity maturation, the final Anticalin PRS-050 was selected to bind all major splice forms of VEGF-A with picomolar affinity. Moreover, this Anticalin cross-reacts with the murine ortholog. PRS-050 efficiently antagonizes the interaction between VEGF-A and its cellular receptors, and it inhibits VEGF-induced mitogenic signaling as well as proliferation of primary human endothelial cells with subnanomolar IC50 values. Intravitreal administration of the Anticalin suppressed VEGF-induced blood–retinal barrier breakdown in a rabbit model. To allow lasting systemic neutralization of VEGF-A in vivo, the plasma half-life of the Anticalin was extended by site-directed PEGylation. The modified Anticalin efficiently blocked VEGF-mediated vascular permeability as well as growth of tumor xenografts in nude mice, concomitantly with reduction in microvessel density. In contrast to bevacizumab, the Anticalin did not trigger platelet aggregation and thrombosis in human FcγRIIa transgenic mice, thus suggesting an improved safety profile. Since neutralization of VEGF-A activity is well known to exert beneficial effects in cancer and other neovascular diseases, including wet age-related macular degeneration, this Anticalin offers a novel potent small protein antagonist for differentiated therapeutic intervention in oncology and ophthalmology.





Similar content being viewed by others
References
Nelson AL, Dhimolea E, Reichert JM (2010) Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9:767–774
Reichert JM (2014) Antibodies to watch in 2014. mAbs 6:5–14
Skerra A (2007) Alternative non-antibody scaffolds for molecular recognition. Curr Opin Biotechnol 18:295–304
Richter A, Eggenstein E, Skerra A (2014) Anticalins: exploiting a non-Ig scaffold with hypervariable loops for the engineering of binding proteins. FEBS Lett 588:213–218
Åkerström B, Borregaard N, Flower DR, Salier J-S (2006) Lipocalins. Landes Bioscience, Georgetown
Skerra A (2000) Lipocalins as a scaffold. Biochim Biophys Acta 1482:337–350
Schiefner A, Skerra A (2015) The menagerie of human lipocalins: a natural protein scaffold for molecular recognition of physiological compounds. Acc Chem Res 48:976–985
Newcomer ME, Jones TA, Aqvist J, Sundelin J, Eriksson U, Rask L, Peterson PA (1984) The three-dimensional structure of retinol-binding protein. EMBO J 3:1451–1454
Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK (2002) The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 10:1033–1043
Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B (2004) Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother 48:3367–3372
Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–921
Beste G, Schmidt FS, Stibora T, Skerra A (1999) Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold. Proc Natl Acad Sci USA 96:1898–1903
Schönfeld D, Matschiner G, Chatwell L, Trentmann S, Gille H, Hülsmeyer M, Brown N, Kaye PM, Schlehuber S, Hohlbaum AM, Skerra A (2009) An engineered lipocalin specific for CTLA-4 reveals a combining site with structural and conformational features similar to antibodies. Proc Natl Acad Sci USA 106:8198–8203
Gebauer M, Schiefner A, Matschiner G, Skerra A (2013) Combinatorial design of an Anticalin directed against the extra-domain B for the specific targeting of oncofetal fibronectin. J Mol Biol 425:780–802
Redl B (2000) Human tear lipocalin. Biochim Biophys Acta 1482:241–248
Breustedt DA, Korndörfer IP, Redl B, Skerra A (2005) The 1.8-Å crystal structure of human tear lipocalin reveals an extended branched cavity with capacity for multiple ligands. J Biol Chem 280:484–493
Breustedt DA, Chatwell L, Skerra A (2009) A new crystal form of human tear lipocalin reveals high flexibility in the loop region and induced fit in the ligand cavity. Acta Crystallogr D Biol Crystallogr 65:1118–1125
Olwill SA, Joffroy C, Gille H, Vigna E, Matschiner G, Allersdorfer A, Lunde BM, Jaworski J, Burrows JF, Chiriaco C, Christian HJ, Hülsmeyer M, Trentmann S, Jensen K, Hohlbaum AM, Audoly L (2013) A highly potent and specific MET therapeutic protein antagonist with both ligand-dependent and ligand-independent activity. Mol Cancer Ther 12:2459–2471
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844
Shojaei F, Ferrara N (2007) Antiangiogenesis to treat cancer and intraocular neovascular disorders. Lab Invest 87:227–230
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3:391–400
Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S (2008) Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300:2277–2285
Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J, Hurwitz H (2007) Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 99:1232–1239
Rudge JS, Holash J, Hylton D, Russell M, Jiang S, Leidich R, Papadopoulos N, Pyles EA, Torri A, Wiegand SJ, Thurston G, Stahl N, Yancopoulos GD (2007) VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci USA 104:18363–18370
Gerber HP, Wu X, Yu L, Wiesmann C, Liang XH, Lee CV, Fuh G, Olsson C, Damico L, Xie D, Meng YG, Gutierrez J, Corpuz R, Li B, Hall L, Rangell L, Ferrando R, Lowman H, Peale F, Ferrara N (2007) Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc Natl Acad Sci USA 104:3478–3483
Meyer T, Robles-Carrillo L, Robson T, Langer F, Desai H, Davila M, Amaya M, Francis JL, Amirkhosravi A (2009) Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost 7:171–181
Christinger HW, Muller YA, Berleau LT, Keyt BA, Cunningham BC, Ferrara N, de Vos AM (1996) Crystallization of the receptor binding domain of vascular endothelial growth factor. Proteins 26:353–357
Skerra A (2001) ‘Anticalins’: a new class of engineered ligand-binding proteins with antibody-like properties. J Biotechnol 74:257–275
Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N (2001) Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 276:3222–3230
Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, Adamis AP (2000) Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol 156:1733–1739
Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, Ferrara N, Adamis AP (1996) Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 103:1820–1828
Narayanan R, Kuppermann BD, Jones C, Kirkpatrick P (2006) Ranibizumab. Nat Rev Drug Discov 5:815–816
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ (2007) Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 114:2179–2182
Roberts MJ, Bentley MD, Harris JM (2002) Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev 54:459–476
Kubetzko S, Sarkar CA, Plückthun A (2005) Protein PEGylation decreases observed target association rates via a dual blocking mechanism. Mol Pharmacol 68:1439–1454
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146:1029–1039
Mordenti J, Thomsen K, Licko V, Chen H, Meng YG, Ferrara N (1999) Efficacy and concentration-response of murine anti-VEGF monoclonal antibody in tumor-bearing mice and extrapolation to humans. Toxicol Pathol 27:14–21
Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM (1998) VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 Å resolution and mutational analysis of the interface. Structure 6:1153–1167
McKenzie SE, Taylor SM, Malladi P, Yuhan H, Cassel DL, Chien P, Schwartz E, Schreiber AD, Surrey S, Reilly MP (1999) The role of the human Fc receptor FcγRIIA in the immune clearance of platelets: a transgenic mouse model. J Immunol 162:4311–4318
Stahl A, Stumpp MT, Schlegel A, Ekawardhani S, Lehrling C, Martin G, Gulotti-Georgieva M, Villemagne D, Forrer P, Agostini HT, Binz HK (2013) Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis 16:101–111
Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, Meng YG, Fei DT, Fourre KM, Ryan AM (1999) Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol 27:536–544
Mross K, Richly H, Fischer R, Scharr D, Buchert M, Stern A, Gille H, Audoly LP, Scheulen ME (2013) First-in-human phase I study of PRS-050 (Angiocal), an Anticalin targeting and antagonizing VEGF-A, in patients with advanced solid tumors. PLoS One 8:e83232
EPAR (2006) Avastin: EPAR—Scientific Discussion. European Medicines Agency; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000582/WC500029262.pdf
Gerber HP, Kowalski J, Sherman D, Eberhard DA, Ferrara N (2000) Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res 60:6253–6258
Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G (2006) Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 281:951–961
Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, Fuh G, Lowman H, Ferrara N (2008) Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci 49:522–527
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611
Akeson A, Herman A, Wiginton D, Greenberg J (2010) Endothelial cell activation in a VEGF-A gradient: relevance to cell fate decisions. Microvasc Res 80:65–74
Finley SD, Engel-Stefanini MO, Imoukhuede PI, Popel AS (2011) Pharmacokinetics and pharmacodynamics of VEGF-neutralizing antibodies. BMC Syst Biol 5:193
Santora LC, Kaymakcalan Z, Sakorafas P, Krull IS, Grant K (2001) Characterization of noncovalent complexes of recombinant human monoclonal antibody and antigen using cation exchange, size exclusion chromatography, and BIAcore. Anal Biochem 299:119–129
Leal T, Robins HI (2010) Bevacizumab induced reversible thrombocytopenia in a patient with recurrent high-grade glioma: a case report. Cancer Chemother Pharmacol 65:399–401
Kumar J, Bhargava M, Aggarwal S (2012) Bevacizumab-induced reversible thrombocytopenia in a patient with adenocarcinoma of colon: rare adverse effect of bevacizumab. Case Rep Oncol Med 2012:695430
Van Walle I, Gansemans Y, Parren PW, Stas P, Lasters I (2007) Immunogenicity screening in protein drug development. Expert Opin Biol Ther 7:405–418
Breustedt DA, Schönfeld DL, Skerra A (2006) Comparative ligand-binding analysis of ten human lipocalins. Biochim Biophys Acta 1764:161–173
Schlehuber S, Beste G, Skerra A (2000) A novel type of receptor protein, based on the lipocalin scaffold, with specificity for digoxigenin. J Mol Biol 297:1105–1120
Kim HJ, Eichinger A, Skerra A (2009) High-affinity recognition of lanthanide(III) chelate complexes by a reprogrammed human lipocalin 2. J Am Chem Soc 131:3565–3576
Skerra A (1994) Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene 151:131–135
Miles AA, Miles EM (1952) Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol 118:228–257
Simpson-Herren L, Lloyd HH (1970) Kinetic parameters and growth curves for experimental tumor systems. Cancer Chemother Rep 54:143–174
Acknowledgments
We are indebted to all current and previous members of the Pieris team, in particular Steffen Schlehuber and Antje Walz, for their contributions and support. We are also grateful to Shane Olwill and Ulrich Moebius for critical reading of the manuscript.
Author contributions
H. G., A. M. H., G. M., M. H., L. P. A. and A. S. designed experiments. H. G., M. H., S. T., G. M., H. J. C. and S. T. performed experiments and analyzed the data. T. M. and A. A. designed, performed and analyzed the experiments in FcγIIa transgenic mice. H. G., A. M. H., L. P. A. and A. S. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
G. M. is a full-time employee at Pieris Pharmaceuticals GmbH. H. G., M. H., S. T., H.-J. C., A. M. H. and L. P. A. were full-time employees of Pieris AG. A. S. is founder of Pieris AG and shareholder of Pieris Pharmaceuticals, Inc.
Ethical standards
All experiments described in this manuscript comply with the laws of the USA and the European Union. All animal experiments were reviewed and approved by the responsible animal ethics committees.
Rights and permissions
About this article
Cite this article
Gille, H., Hülsmeyer, M., Trentmann, S. et al. Functional characterization of a VEGF-A-targeting Anticalin, prototype of a novel therapeutic human protein class. Angiogenesis 19, 79–94 (2016). https://doi.org/10.1007/s10456-015-9490-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-015-9490-5