Abstract
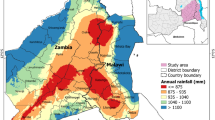
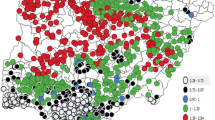
Malaria in South Africa is still a problem despite existing efforts to eradicate the disease. In the Vhembe District Municipality, malaria prevalence is still high, with a mean incidence rate of 328.2 per 100,0000 persons/year. This study aimed at evaluating environmental covariates, such as vegetation moisture and vegetation greenness, associated with malaria vector distribution for better predictability towards rapid and efficient disease management and control. The 2005 malaria incidence data combined with Landsat 5 ETM were used in this study. A total of nine remotely sensed covariates were derived, while pseudo-absences in the ratio of 1:2 (presence/absence) were generated at buffer distances of 0.5–20 km from known presence locations. A stepwise logistic regression model was applied to analyse the spatial distribution of malaria in the area. A buffer distance of 10 km yielded the highest classification accuracy of 82% at a threshold of 0.9. This model was significant (ρ < 0.05) and yielded a deviance (D2) of 36%. The significantly positive relationship (ρ < 0.05) between the soil-adjusted vegetation index and malaria distribution at all buffer distances suggests that malaria vector (Anopheles arabiensis) prefer productive and greener vegetation. The significant negative relationship between water/moisture index (a1 index) and malaria distribution in buffer distances of 0.5, 10, and 20 km suggest that malaria distribution increases with a decrease in shortwave reflectance signal. The study has shown that suitable habitats of malaria vectors are generally found within a radius of 10 km in semi-arid environments, and this insight can be useful to aid efforts aimed at putting in place evidence-based preventative measures against malaria infections. Furthermore, this result is important in understanding malaria dynamics under the current climate and environmental changes. The study has also demonstrated the use of Landsat data and the ability to extract environmental conditions which favour the distribution of malaria vector (An. arabiensis) such as the canopy moisture content in vegetation, which serves as a surrogate for rainfall.








Similar content being viewed by others
Abbreviations
- AIC:
-
Akaike’s information criterion
- ASTER:
-
Advanced Spaceborne Thermal Emission and Reflection Radiometer
- D 2 :
-
Deviance
- DEM:
-
Digital elevation model
- GARP:
-
Genetic Algorithm for Rule Set Production
- GI:
-
Greenness index
- MIS:
-
Malaria information system
- MNDWI:
-
Modified normalized difference water index
- NDVI:
-
Normalized difference vegetation index
- p-YI:
-
Quasi-yellowness index
- SAVI:
-
Soil-adjusted vegetation index
- SDM:
-
Species distribution modelling
- SLR:
-
Stepwise logistic regression
- TM:
-
Thematic Mapper
- VDM:
-
Vhembe District Municipality
References
Adams J (2010) Vegetation–Climate Interaction. How Plants Make the Global Environment, 2nd ed., New York: Springer. ISBN:978-3-642-00880-1.
Adams M.L., Philpot W.D., Norvell W.A. (1999). Yellowness index: An application of spectral second derivatives to estimate chlorosis of leaves in stressed vegetation. International Journal of Remote Sensing 20: 3663–3675.s.
Adeola A.M., Botai J.O., Olwoch J.M., Rautenbach H.C.J., Kalumba A.M., Tsela P.L., Adisa M.O., Wasswa N.F., Mmtoni P., Ssentongo A. (2015). Application of geographical information system and remote sensing in malaria research and control in South Africa: a review. Southern African Journal of Infectious Diseases 1: 1 – 8.
Adeola A.M., Botai O.J., Olwoch J.M., Rautenbach C.J., Adisa O.M., Taiwo O.J., Kalumba A.M. (2016). Environmental factors and population at risk of malaria in Nkomazi municipality, South Africa. Tropical Medicine and International Health 21(5):675–686.
Adimi F., Soebiyanto R.P., Safi N., Kiang R. (2010). Towards malaria risk prediction in Afghanistan using remote sensing. Malaria Journal 9:125.
Ahmed A (2014) GIS and remote sensing for malaria risk mapping, Ethiopia. The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, ISPRS Technical Commission VIII Symposium, 09–12 Dec 2014, Hyderabad, India 8: 155–161.
Alimi T., Fuller D.O., Qualls W.A., Herrera S.V., Arevalo-Herrera, Quinones M.L., Lacerda M.V.G., Beier J.C. (2015). Predicting potential ranges of primary malaria vectors and malaria in northern South America based on projected changes in climate, land cover and human population. Parasites and Vectors 8: 1 – 16.
Araujo S.L., Santos J.R., Shimabukuro Y.E. (2000). Relationship between SAVI and biomass data of forest and savannah contact zone in the Brazillian Amozonia. International Archives of Photogrammetry and Remote Sensing XXXIII:77–81.
Baeza A., Bouma M.J., Bobson A.P., Dhiman R., Srivastava H.C., Mascual M. (2011). Climate forcing desert malaria: the effect of irrigation. Malaria Journal 10 (90):1 – 10.
Baldwin R.A. (2009). Use of maximum entropy modelling in wildlife research. Entropy 11:854–866.
Barbet-Massin M., Jiguet F., Albert C.H., Thuiller W. (2012). Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution 3:327–338.
Bennie J., Hill M.O., Baxter R., Huntley B. (2006). Influence of slope and aspect on long-term vegetation change in British chalk grasslands. Journal of Ecology 94: 355 – 368.
Berkson J. (1938). Some difficulties of interpretation encountered in the application of the Chi square test. Journal of the American Statistical Association 33: 526 – 542.
Bernstein LS, Adler-Golden S, Jin X, Gregor B (2012) Quick atmospheric correction (QUAC) code for VNIR-SWIR spectral imagery: algorithm details. The 4th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Shanghai, pp 1–4.
Bowman W.D. (1989). The relationship between leaf water status, gas exchange and spectral reflectance in cotton leaves. Remote Sensing of Environment 30(3): 249 – 255.
Busby J.R. (1991) BIOCLIM—a bioclimate analysis and prediction system. Plant Protection Quarterly 6:8–90
Calcagno V., de Mazancourt C. (2010). glmulti: An R Package for Easy Automated Model Selection with (Generalized) Linear Models. Journal of Statistical Software 34: 1 – 29.
Ceccato P., Flasse S., Gregoire J. (2002). Designing a spectral index to estimate vegetation water content from remote sensing data: Part 2. Validation and applications. Remote Sensing of Environment 82: 198 – 207.
Ceccato P., Flasse S., Tarantola S., Jacquemoud S., Gregoire J. (2001). Detecting vegetation leaf water content using reflectance in the optical domain. Remote Sensing of Environment 77: 22 – 33.
Chefaoui R.M., Lobo J.M. (2008). Assessing the effects of pseudo-absences on predictive distribution model performance. Ecological ModelingModelling 210: 478 – 486.
Clennon J.A., Kamanga A., Musapa M., Shiff C., Glass G.E. (2010). Identifying malaria vector breeding habitats with remote sensing data and terrain-based landscape indices in Zambia. International Journal of Health Geographics 9 (58): 1 – 13.
Collett D. (1991). Modeling binary data. Chapman and Hall, London.
Craig M.H., Snow R.W., Le Sueur D. (1999). A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitology Today 15(3): 105 – 111.
Dambach P., Machault V., Lacaux J., Vignolles C., Sauerborn R. (2012). Utilization of combined remote sensing techniques to detect environmental variables influencing malaria vector densities in rural West Africa. International Journal of Health Geographics 11(8): 1 – 12.
De Oliveira E.C., do Santos E.S., Zeilhofer P., Souza-Santos R., Atanaka-Santos M. (2013). Geographic information systems and logistic regression for high-resolution malaria risk mapping in a rural settlement of the southern Brazilian Amazon. Malaria Journal 12:420.
Dlamini SN, Franke J, Vounatsou P (2015).Assessing the relationship between environmental factors and malaria vector breeding sites in Swaziland using multi-scale remotely sensed data. Geospatial Health. https://doi.org/10.4081/gh.2015.302.
Exelis Visual Information Solutions (2016) Environment for visualizing images. Boulder, CO: Exelis Visual Information Solutions.
Ganser C., Wisely S.M. (2013). Patterns of spatio-temporal distribution, abundance, and diversity in a mosquito community from the eastern Smoky Hills of Kansas. Journal of Vector Ecology 38(2): 229 – 236.
Gao B. (1996). NDWI – a normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sensing of Environment 58: 257 – 266.
Gerritsen A.A.M., Kruger P., Schim van der Loeff M.F., Grobusch M.P. (2008). Malaria incidence in Limpopo Province, South Africa, 1998 – 2007. Malaria Journal 7:1 – 8.
Gitelson A.A. (2003). Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. Journal of Plant Physiology 160: 271 – 282.
Gomez-Elipe A., Otero A., van Herp M., Aguire-Jaime A. (2007). Forecasting malaria incidence based on monthly case reports and environmental factors in Karuzi, Burundi, 1997–2003. Malaria Journal. https://doi.org/10.1186/1475-2875-6-129.
Huete A.R. (1988). A soil-adjusted vegetation index (SAVI). Remote Sensing of Environment 25(3):295–309.
Hunt J.R., Rock B.N. (1989). Detection of changes in leaf water content using near-infrared and middle-infrared reflectances. Remote Sensing of Environment 30: 43 – 54.
Ikeda T., Behera S.K., Morioka Y., Minakawa N., Hashizume M., Tsuzuki A., Maharaj R., Kruger P. (2017). Seasonally lagged effects of climatic factors on malaria incidence in South Africa. Scientific Reports 7(2458):1–9. (https://doi.org/10.1038/s41598-017-02680-6).
Jackson R.D, Slater P.N, Pinter P.J. (1983). Discrimination of growth and water stress in wheat by various vegetation indices through clear and turbid atmospheres. Remote Sensing of Environment 13:187–208.
Jacob B.G., Muturi E.J., Mwangangi J.M., Funes J., Caamano E.X., Muriu S., Shililu J., Guthure J., Novak R.J. (2007). Remote and field level quantification of vegetation covariates for malaria mapping in three rice agro-village complexes in Central Kenya. International Journal of Health Geographics. https://doi.org/10.1186/1476-072X-6-21.
Kabanda T, Munyati C (2010) Anthropogenic-induced climate change and the resulting tendency to land conflict; The case of the Soutpansberg region, South Africa; Climate Change and Natural Resources Conflicts in Africa. In: Monograph No 170: Institute for Security Studies, Donald Anthony Mwiturubani, Jo-Ansie van Wyk (editors).
Kamau L., Munyekenye G.O., Vulule J.M., Lehmann T. (2006). Evaluating genetic differentiation of Anopheles arabiensis in relation to larval habitats in Kenya. Infections, Genetics and Evolution 7: 293 – 297.
Kazembe L.N., Kleinschmidt I., Holtz T.H., Sharp B.L. (2006). Spatial analysis and mapping of malaria risk in Malawi using point-referenced prevalence of infection data. International Journal of Health Geographics 5(41): 1 – 9.
Khosa E., Kuonza L.R., Kruger P., Maimela E. (2013). Towards the elimination of malaria in South Africa: a review of surveillance data in Mutale Municipality, Limpopo Province, 2005 to 2010. Malaria Journal. https://doi.org/10.1186/1475-2875-12-7.
Kleinschmidt I., Bagayoko M., Clarke G.P.Y., Graig M., Le Sueur D. (2000). A spatial statistical approach to malaria mapping. International Journal of Epidemiology 29: 355 – 361.
Komen K. (2017). Could Malaria Control Programmes be timed to coincide with onset of rainfall? EcoHealth 14(2): 259 – 271.
Komen K., Olwoch J., Rautenbach H., Botai J., Adebayo A. (2015). Long-run relative importance of temperature as the main driver to malaria transmission in Limpopo Province, South Africa: a smiple econometric approach. EcoHealth 12(1): 131 – 143.
Lopatin J., Fassnacht F.E., Kattenborn T., Schmidtlein S. (2017). Mapping plant species in mixed grassland communities using close range imaging spectroscopy. Remote Sensing of Environment 201:12–23.
Mabaso M.L.H., Craig M., Vounatsou P., Smith T. (2005). Towards empirical description of malaria seasonality in southern Africa: the example if Zimbabwe. Tropical Medicine and International Health 10(9): 909 – 918.
Mabaso M.L.H., Vounatsou P., Midzi S., Da Silva J., Smith T. (2006). Spatio-temporal analysis of the role of climate in inter-annual variation of malaria incidence in Zimbabwe. International Journal of Health Geographics 5(20): 1 – 9.
Machault V., Vignolles C., Borchi F., Vounatsou P., Pages F., Briolant S., lacaux J., Rogier C. (2011). The use of remotely sensed environmental data in the study of malaria. Geospatial Health 5: 151 – 168.
Machault V., Vignolles C., Pagès F., Gadiaga L., Gaye A., Sokhna C., Trape J., Lacaux J., Rogier C. (2010). Spatial heterogeneity and temporal evolution of malaria transmission risk in Dakar, Senegal, according to remotely sensed environmental data. Malaria Journal 9(252): 1 – 14.
Maharaj R., Morris N., Seocharan I., Kruger P., Moonasar D., Mabuza A., Raswiswi E., Raman J. (2012). The feasibility of malaria elimination in South Africa. Malaria Journal. https://doi.org/10.1186/1475-2875-11-423.
Malahlela O, Cho M.A., Mutanga O. (2014). Mapping canopy gaps in an indigenous subtropical coastal forest using high resolution WorldView-2 data. International Journal of Remote Sensing 35: 6397–6417.
Mendis K., Ritveld A., Warsame M., Bosman A., Greenwood B., Wernsdorfer W.H. (2009). From malaria control to eradication: the WHO perspective. Tropical Medicine and International Health 14(7):802–809.
Mpandeli S. (2014). Managing Climate Risks Using Seasonal Climate Forecast Information in Vhembe District in Limpopo Province, South Africa. Journal of Sustainable Development 7 (5): 68 – 81.
National Department of Health (2011).Malaria Elimination Strategy 2011–2018 in South Africa. South Africa: National Department of Health (NDoH).
National Institute for Communicable Diseases (NICD)-National Health Laboratory Services (NHLS) (2017) Malaria in South Africa: an update 16(5):1.
Nmor J.C., Sunahara T., Goto K., Futami K., Sonye G., Akweywa P., Dida G., Minakawa N. (2013). Topographic models for predicting malaria vector breeding habitats: potential tools for vector control managers. Parasites and Vectors 6: 1 – 13.
Omumbo J.A., Hay S.I., Goetz S.J., Snow R.W., Rogers D.J. (2002). Updating Historical Maps of Malaria Transmission Intensity in East Africa Using Remote Sensing. Photogrammetric Engineering &Remote Sensing 68: 161 – 166.
Phillips S.J., Anderson R.P., Schapire R.E. (2006). Maximum entropy modelling of species geographic distributions. Ecological Modelling 190: 231–259.
Phillips S.J., Dudík M., Elith J., Graham C.H., Lehmann A., Leathwick J., Ferrier S. (2009). Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecological Applications 19:181 – 197.
Protopopoff N., van Bortel W., Speybroeck N., van Geertruyden J., Baza D., D’Alessandro U., Coosemans M. (2009). Ranking Malaria Risk Factors to Guide Malaria Control Efforts in African Highlands. PLoS One:. https://doi.org/10.1371/journal.pone.0008022.
QGIS Development Team (2016) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
Raman J, Morris N, Frean J, Brooke B., Blumberg L., Kruger P., Mabusa A., Raswiswi E., Shandukani B., Misani E., Groepe M., Moonasar D (2016) Reviewing South Africa’s malaria elimination strategy (2012-2018): progress, challenges and priorities. Malaria Journal 15:438.
Reason C.J.C., Keibel A. (2004). Tropical cyclone Eline and its unusual penetration and impacts over the southern African mainland. Weather Forecast 19(5): 789 – 805.
Ricotta E.E., Frese S.A., Choobwe C., Louis T.A., Shiff C.J. (2014). Evaluating local vegetation cover as a risk factor for malaria transmission: a new analytical approach using ImageJ. Malaria Journal. https://doi.org/10.1186/1475-2875-13-94.
Ripley BD (2003) Selecting amongst large classes of models. Lecture, URL http://www.stats.ox.ac.uk/~ripley/Nelder80.pdf.
Rossiter DG, Loza A (2016) Technical note: analysing land cover change with logistic regression in R. 1 – 67. http://www.css.cornell.edu/faculty/dgr2/teach/R/R_lcc.pdf. Accessed on the 30/10/2017.
Sinka M.E., Rubio-Palis Y., Manguin S., Patil A.P., Temperly W.H., Gething P.W., Van Boeckel T., Kabaria C.W., Harbach R.E., Hay S.I. (2010). The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic précis. Parasites and Vectors 3: 1 – 26.
Statistics South Africa (2016) Population census 2016. Vhembe District Municipality. http://cs2016.statssa.gov.za/?page_id=270.
Stockwell D.R.B., Peters D.P. (1999). The GARP modelling system: problems and solutions to automated spatial prediction. International Journal of Geographic Information Systems 13:143–158.
Tonnang H.E.Z., Kangalawe R.Y.M., Yanda P.Z. (2010). PreictingPredicting and mapping malaria under climate change scenarios: the potential distribution of malaria vectors in Africa. Malaria Journal 9(111): 1 – 10.
Tucker C.J. (1980). Remote sensing of leaf water content in the near infrared. Remote Sensing of Environment 10(1): 23 – 32.
Wayant N.M., Maldonado D., Rojas de Aria A., Cousiño B., Goodin D.G. (2010). Correlation between normalized difference vegetation index and malaria in a subtropical rain forest undergoing rapid anthropogenic alteration. Geospatial Health 4: 179 – 190.
World Health Organization (WHO) (2016) World malaria report 2016. Geneva: World Licence:CC BY-NC-SA 3.0 IGO.
Xu H. (2006). Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. International Journal of Remote Sensing 27(14):3025–3033.
Zhou G., Minakawa N., Githeko A.K., Yan G. (2004). Association between climate variability and malaria epidemics in the East African highlands. Proceedings of the National Academy of Sciences of the United States of America 101(8): 2375 – 2380.
Zhou S., Zhang S., Wang J., Zheng X., Huang F., Li W., Zhang H. (2012). Spatial correlation between malaria cases and water-bodies in Anopheles sinensis dominated areas of Huang-Huai plain, China. Parasites and Vectors 5(106):1 – 7.
Acknowledgements
Authors would like to thank QGIS Development Team for the Quantum GIS software used in this study. We would like to also extent our gratitude to the R Development Team for making R software available for data analysis. Our gratitude to the USGS for free Landsat dataset used in the study. This work is funded by the South African National Space Agency under the Human Capital Development. Authors would also like to thank two anonymous reviewers who have helped improve the quality of this manuscript.
Author information
Authors and Affiliations
Contributions
OM, JO, CA conceptualized the research. JO and CA participated in data analysis. OM drafted the manuscript. JO and CA supervised the entire work. All authors read and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Malahlela, O.E., Olwoch, J.M. & Adjorlolo, C. Evaluating Efficacy of Landsat-Derived Environmental Covariates for Predicting Malaria Distribution in Rural Villages of Vhembe District, South Africa. EcoHealth 15, 23–40 (2018). https://doi.org/10.1007/s10393-017-1307-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-017-1307-0