Abstract
The necessity to develop automated methods for the fast screening of new libraries of compounds and the identification of active entities from natural mixtures has led to an increasing interest in the development of immobilized enzyme reactors (IMERs). This strategy overcomes some drawbacks of the in-solution methods and is, therefore, very attractive in the drug discovery field. This review gives an overview of IMER applications in the last decade. The reported examples concern conventional columns as well as capillary reactors integrated in liquid chromatography or capillary electrophoresis systems, coupled to spectroscopic or mass spectrometry detectors. The experimental setups and main features as well as characterization of new active entities are discussed. As a result of the growing importance of compounds from natural sources in drug discovery, particular attention is given to IMERs developed to be used for the identification of bioactive compounds.
Graphical Abstract



Reprinted with permission from J. Pharm. Biomed. Anal. [33]

Reprinted with permission from J. Chromatogr. B. [36]


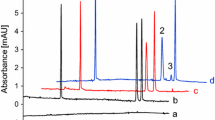
Reprinted with permission from Anal. Biochem. [66]

Reprinted with permission from [80]
Similar content being viewed by others
References
Bertucci C, Bartolini M, Gotti R, Andrisano V (2003) J Chromatogr B Analyt Technol Biomed Life Sci 797:111–129
Girelli AM, Mattei E (2005) J Chromatogr B Analyt Technol Biomed Life Sci 819:3–16. https://doi.org/10.1016/j.jchromb.2005.01.031
Jason-Moller L, Murphy M, Bruno J (2006) Curr Protoc Protein Sci 45:19.13.1–19.13.14. https://doi.org/10.1002/0471140864.ps1913s45
Choi JW, Oh BK, Kim YK, Min J (2007) J Microbiol Biotechnol 17:5–14
Lee J, Soper SA, Murray KK (2009) Anal Chim Acta 649:180–190. https://doi.org/10.1016/j.aca.2009.07.037
Krenková J, Foret F (2004) Electrophoresis 25:3550–3563. https://doi.org/10.1002/elps.200406096
Fang SM, Wang HN, Zhao ZX, Wang WH (2012) J Pharm Anal 2:83–89. https://doi.org/10.1016/j.jpha.2011.12.002
Brena BM, Irazoqui G, Giacomini C, Batista-Viera F (2003) Effect of increasing co-solvent concentration on the stability of soluble and immobilized beta-galactosidase. J Mol Catal B Enzym 21:25–29
Schejbal J, Glatz Z (2018) J Sep Sci 41:323–335. https://doi.org/10.1002/jssc.201700905
Vilanova E, Manjon A, Iborra JL (1984) Biotechnol Bioeng 26:1306–1312. https://doi.org/10.1002/bit.260261107
Luckarift HR, Johnson GR, Spain JC (2006) J Chromatogr B Analyt Technol Biomed Life Sci 843:310–316. https://doi.org/10.1016/j.jchromb.2006.06.036
Hu F, Deng C, Zhang X (2008) J Chromatogr B Analyt Technol Biomed Life Sci 871:67–71. https://doi.org/10.1016/j.jchromb.2008.06.036
Freije R, Klein T, Ooms B, Kauffman HF, Bischoff R (2008) J Chromatogr A 1189:417–425. https://doi.org/10.1016/j.chroma.2007.10.059
Wu S, Sun L, Ma J, Yang K, Liang Z, Zhang L, Zhang Y (2011) Talanta 83:1748–1753. https://doi.org/10.1016/j.talanta.2010.12.011
Fossati T, Colombo M, Castiglioni C, Abbiati G (1994) J Chromatogr B Biomed Appl 656:59–64
Yamato S, Kawakami N, Shimada K, Ono M, Idei N, Itoh Y, Tachikawa E (2004) Biol Pharm Bull 27:210–215
Shu HC, Wu NP (2001) Talanta 54:361–368
Markoglou N, Wainer IW (2002) J Chromatogr A 948:249–256
Mattiasson B (1988) Methods Enzymol 137:647–656
Gast FU, Franke I, Meiss G, Pingoud A (2001) J Biotechnol 87:131–141
Luckarift HR, Ku BS, Dordick JS, Spain JC (2007) Biotechnol Bioeng 98:701–705. https://doi.org/10.1002/bit.21447
Betancor L, Luckarift HR (2008) Trends Biotechnol 26:566–572. https://doi.org/10.1016/j.tibtech.2008.06.009
Subramanian A, Kennel SJ, Oden PI, Jacobson KB, Woodward J, Doktycz MJ (1999) Comparison of techniques for enzyme immobilization on silicon supports. Enzyme Microbial Technol 24:26
He P, Greenway G, Haswell SJ (2008) Nanotechnology 19:315603. https://doi.org/10.1088/0957-4484/19/31/315603
Kim D, Herr AE (2013) Biomicrofluidics 7:41501. https://doi.org/10.1063/1.4816934
Andrisano V, Bartolini M (2010) Immobilisation of enzymes on monolithic matrices: applications in drug discovery. In: Wang PG (eds) Monolithic chromatography and its modern applications. ILM, London
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Soc 56:658–666
Dixon M (1953) The determination of enzyme inhibitor constants. Biochem J 55:170–171
Cornish-Bowden A (1974) A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J 137:143–144
Vodopivec M, Podgornik A, Berovic M, Strancar A (2003) J Chromatogr B Analyt Technol Biomed Life Sci 795:105–113
Wheatley JB, Schmidt DE (1999) J Chromatogr A 849:1–12
Bartolini M, Cavrini V, Andrisano V (2005) J Chromatogr A 1065:135–144
De Simone A, Mancini F, Cosconati S, Marinelli L, La Pietra V, Novellino E, Andrisano V (2013) J Pharm Biomed Anal 73:131–134. https://doi.org/10.1016/j.jpba.2012.03.006
Andrisano V, Bartolini M, Gotti R, Cavrini V, Felix G (2001) J Chromatogr B Biomed Sci Appl 753:375–383
Chlebek J, De Simone A, Hošťálková A, Opletal L, Pérez C, Pérez DI, Havlíková L, Cahlíková L, Andrisano V (2016) Fitoterapia 109:241–247. https://doi.org/10.1016/j.fitote.2016.01.008
De Simone A, Seidl C, Santos CA, Andrisano V (2014) J Chromatogr B Analyt Technol Biomed Life Sci 953–954:108–114. https://doi.org/10.1016/j.jchromb.2014.01.056
Mancini F, Andrisano V (2010) J Pharm Biomed Anal 52:355–361. https://doi.org/10.1016/j.jpba.2009.07.012
Mancini F, Naldi M, Cavrini V, Andrisano V (2007) J Chromatogr A 1175:217–226. https://doi.org/10.1016/j.chroma.2007.10.047
Mancini F, De Simone A, Andrisano V (2011) Anal Bioanal Chem 400:1979–1996. https://doi.org/10.1007/s00216-011-4963-x
Seidl C, de Moraes Santos CA, De Simone A, Bartolini M, Weffort-Santos AM, Andrisano V (2017) Curr Alzheimer Res 14:317–326. https://doi.org/10.2174/1567205013666161026150455
Bartolini M, Greig NH, Yu QS, Andrisano V (2009) J Chromatogr A 1216:2730–2738. https://doi.org/10.1016/j.chroma.2008.09.100
Nicoli R, Bartolini M, Rudaz S, Andrisano V, Veuthey JL (2008) J Chromatogr A 1206:2–10. https://doi.org/10.1016/j.chroma.2008.05.080
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) Biochem Pharmacol 7:88–95
Perola E, Cellai L, Lamba D, Filocamo L, Brufani M (1997) Biochim Biophys Acta 1343:41–50
André C, Herlem G, Gharbi T, Guillaume YC (2011) J Pharm Biomed Anal 55:48–53. https://doi.org/10.1016/j.jpba.2011.01.003
Morris SM (2002) Annu Rev Nutr 22:87–105. https://doi.org/10.1146/annurev.nutr.22.110801.140547
Wu G, Meininger CJ (1995) Am J Physiol 269:H1312–H1318. https://doi.org/10.1152/ajpheart.1995.269.4.H1312
Kuhn NJ, Ward S, Piponski M, Young TW (1995) Arch Biochem Biophys 320:24–34. https://doi.org/10.1006/abbi.1995.1338
Raman NN, Khan M, Hasan R (1994) Bioactive components from Ficus glomerata. Pure Appl Chem 66:2287–2290
Vanzolini KL, Vieira LC, Corrêa AG, Cardoso CL, Cass QB (2013) J Med Chem 56:2038–2044. https://doi.org/10.1021/jm301732a
Vilela AF, da Silva JI, Vieira LC, Bernasconi GC, Corrêa AG, Cass QB, Cardoso CL (2014) J Chromatogr B Analyt Technol Biomed Life Sci 968:87–93. https://doi.org/10.1016/j.jchromb.2013.11.037
da Silva JI, de Moraes MC, Vieira LC, Corrêa AG, Cass QB, Cardoso CL (2013) J Pharm Biomed Anal 73:44–52. https://doi.org/10.1016/j.jpba.2012.01.026
Orhan IE (2012) Curr Med Chem 19:2252–2261
Anand N, Singh P, Sharma A, Tiwari S, Singh V, Singh DK, Srivastava KK, Singh BN, Tripathi RP (2012) Bioorg Med Chem 20:5150–5163. https://doi.org/10.1016/j.bmc.2012.07.009
Catto M, Pisani L, Leonetti F, Nicolotti O, Pesce P, Stefanachi A, Cellamare S, Carotti A (2013) Bioorg Med Chem 21:146–152. https://doi.org/10.1016/j.bmc.2012.10.045
Peng XM, Damu GL, Zhou C (2013) Curr Pharm Des 19:3884–3930
Nordberg A, Ballard C, Bullock R, Darreh-Shori T, Somogyi M (2013) Prim Care Companion CNS Disord 15:1–8. https://doi.org/10.4088/pcc.12r01412
Kruskal WH, Wallis WA (1952) J Am Statist Assoc 47:583–621
Cornelio VE, de Moraes MC, Domingues VC, Fernandes JB, da Silva MFDG, Cass QB, Vieira PC (2018) J Pharm Biomed Anal 151:252–259. https://doi.org/10.1016/j.jpba.2018.01.001
Benes P, Vetvicka V, Fusek M (2008) Crit Rev Oncol Hematol 68:12–28. https://doi.org/10.1016/j.critrevonc.2008.02.008
Cardoso CL, Lima VV, Zottis A, Oliva G, Andricopulo AD, Wainer IW, Moaddel R, Cass QB (2006) J Chromatogr A 1120:151–157. https://doi.org/10.1016/j.chroma.2005.10.063
de Moraes MC, Ducati RG, Donato AJ, Basso LA, Santos DS, Cardoso CL, Cass QB (2012) J Chromatogr A 1232:110–115. https://doi.org/10.1016/j.chroma.2011.10.056
Galmarini CM (2006) IDrugs 9:712–722
Kalckar HM (1947) J Biol Chem 167:429–443
Bartolini M, Cavrini V, Andrisano V (2007) J Chromatogr A 1144:102–110. https://doi.org/10.1016/j.chroma.2006.11.029
Vilela AFL, Seidl C, Lima JM, Cardoso CL (2018) Anal Biochem 549:53–57. https://doi.org/10.1016/j.ab.2018.03.012
Darvesh S, Walsh R, Kumar R, Caines A, Roberts S, Magee D, Rockwood K, Martin E (2003) Alzheimer Dis Assoc Disord 17:117–126
Forsberg EM, Green JR, Brennan JD (2011) Anal Chem 83:5230–5236. https://doi.org/10.1021/ac200534t
Forsberg EM, Brennan JD (2014) Anal Chem 86:8457–8465. https://doi.org/10.1021/ac5022166
Besanger TR, Hodgson RJ, Green JR, Brennan JD (2006) Anal Chim Acta 564:106–115. https://doi.org/10.1016/j.aca.2005.12.066
La Motta C, Sartini S, Mugnaini L, Salerno S, Simorini F, Taliani S, Marini AM, Da Settimo F, Lavecchia A, Novellino E, Antonioli L, Fornai M, Blandizzi C, Del Tacca M (2009) J Med Chem 52:1681–1692. https://doi.org/10.1021/jm801427r
Alunni S, Orrù M, Ottavi L (2008) J Enzyme Inhib Med Chem 23:182–189. https://doi.org/10.1080/14756360701475233
Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, Camaioni E (2001) Med Res Rev 21:105–128
de Moraes MC, Temporini C, Calleri E, Bruni G, Ducati RG, Santos DS, Cardoso CL, Cass QB, Massolini G (2014) J Chromatogr A 1338:77–84. https://doi.org/10.1016/j.chroma.2014.02.057
Brekkan E, Lundqvist A, Lundahl P (1996) Biochemistry 35:12141–12145. https://doi.org/10.1021/bi9603231
Haneskog L, Lundqvist A, Lundahl P (1998) J Mol Recognit 11:58–61. https://doi.org/10.1002/(SICI)1099-1352(199812)11:1/6%3c58:AID-JMR390%3e3.0.CO;2-S
Haneskog L, Zeng CM, Lundqvist A, Lundahl P (1998) Biochim Biophys Acta 1371:1–4
Ouimet CM, D’amico CI, Kennedy RT (2017) Expert Opin Drug Discov 12:213–224. https://doi.org/10.1080/17460441.2017.1268121
Iqbal J, Iqbal S, Müller CE (2013) Analyst 138:3104–3116. https://doi.org/10.1039/c3an00031a
Ji X, Ye F, Lin P, Zhao S (2010) Talanta 82:1170–1174. https://doi.org/10.1016/j.talanta.2010.06.029
Haynes J, Killilea DW, Peterson PD, Thompson WJ (1996) J Pharmacol Exp Ther 276:752–757
Lin P, Zhao S, Lu X, Ye F, Wang H (2013) J Sep Sci 36:2538–2543. https://doi.org/10.1002/jssc.201300315
Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V (2016) Int J Cardiol 213:8–14. https://doi.org/10.1016/j.ijcard.2015.08.109
Kim SC, Schneeweiss S, Choudhry N, Liu J, Glynn RJ, Solomon DH (2015) Am J Med 128:616–657. https://doi.org/10.1016/j.amjmed.2015.01.013
Zhang L, Hu K, Li X, Zhao S (2018) CE method with partial filling techniques for screening of xanthine oxidase inhibitor in traditional Chinese medicine. Chromatographia 73:583–587
Iqbal J (2011) Anal Biochem 414:226–231. https://doi.org/10.1016/j.ab.2011.03.021
Lanier M, Sergienko E, Simão AM, Su Y, Chung T, Millán JL, Cashman JR (2010) Bioorg Med Chem 18:573–579. https://doi.org/10.1016/j.bmc.2009.12.012
Teriete P, Pinkerton AB, Cosford ND (2013) Methods Mol Biol 1053:85–101. https://doi.org/10.1007/978-1-62703-562-0_5
Narisawa S, Harmey D, Yadav MC, O’Neill WC, Hoylaerts MF, Millán JL (2007) J Bone Miner Res 22:1700–1710. https://doi.org/10.1359/jbmr.070714
Wang S, Su P, Yang Y (2012) Anal Biochem 427:139–143. https://doi.org/10.1016/j.ab.2012.05.014
Guascito MR, Malitesta C, Mazzotta E, Turco A (2008) Inhibitive determination of metal ions by an amperometric glucose oxidase biosensor: study of the effect of hydrogen peroxide decomposition. Sensors Actuators B Chem 131:394–402
Jiang TF, Liang TT, Wang YH, Zhang WH, Lv ZH (2013) J Pharm Biomed Anal 84:36–40. https://doi.org/10.1016/j.jpba.2013.05.023
Gao X, Luo W, Xie G, Xue C, Ding Q (2004) Characteristics and kinetics of inhibition of polyphenol oxidase from Spodoptera exigua (Lepidoptera: Noctuidae). Sci Agric Sin:687–691
Camara MA, Tian M, Guo L, Yang L (2015) J Chromatogr B Analyt Technol Biomed Life Sci 990:174–180. https://doi.org/10.1016/j.jchromb.2015.03.019
Ham M, Choe SS, Shin KC, Choi G, Kim JW, Noh JR, Kim YH, Ryu JW, Yoon KH, Lee CH, Kim JB (2016) Diabetes 65:2624–2638. https://doi.org/10.2337/db16-0060
Zhang C, Zhang Z, Zhu Y, Qin S (2014) Anticancer Agents Med Chem 14:280–289
Schejbal J, Řemínek R, Zeman L, Mádr A, Glatz Z (2016) J Chromatogr A 1437:234–240. https://doi.org/10.1016/j.chroma.2016.01.081
Funding
The authors receive no funds to develop this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Published in the topical collection Rising Stars in Separation Science, as part of Chromatographia’s 50th Anniversary Commemorative Issue.
Rights and permissions
About this article
Cite this article
De Simone, A., Naldi, M., Bartolini, M. et al. Immobilized Enzyme Reactors: an Overview of Applications in Drug Discovery from 2008 to 2018. Chromatographia 82, 425–441 (2019). https://doi.org/10.1007/s10337-018-3663-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3663-5