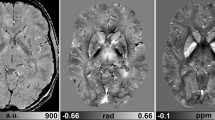
Abstract
Purpose
Objects that cause a susceptibility gradient can generate regions of hypo-intensity in MRI. MR techniques developed for positive enhancement of such objects require sequence parameter optimization. Thus comparison of images acquired successively using different techniques is difficult since different parameter settings result in variations in signal and noise. A new method is presented that allows production of positive contrast images, a relaxation rate \({{\rm R}_{2}^{\ast}}\)-map and negative contrast images from a single dataset by post-processing.
Methods
Positive contrast techniques considered include the “white marker” technique, inversion-recovery on-resonance (IRON) and susceptibility gradient mapping (SGM). The new method was tested in phantoms of iron-oxide agent gel solutions and prostate marker seeds. Images produced by post-processing were compared with those obtained directly. The post-processing technique was applied in vivo for the visualization of iron-oxide contrast agent uptake in a balloon-injured swine carotid model.
Results
The images produced in the post-processing step allowed determination of optimal parameter settings for each technique. SGM was found to provide the greatest positive contrast, whilst the \({{\rm T}_{2}^{\ast}}\)-weighted images provide more sensitivity to regions that exhibited weaker susceptibility effects.
Conclusions
Combined \({{\rm T}_{2}^{\ast}}\)-weighted imaging and SGM using the same complex image data was found to provide complementary information and high sensitivity to detect distortion inducing agents.
Similar content being viewed by others
References
Wuerfel J, Tysiak E, Prozorovski T, Smyth M, Mueller S, Schnorr J, Taupitz M, Zipp F (2007) Mouse model mimics multiple sclerosis in the clinico-radiological paradox. Eur J Neurosci 26: 190–198
Heymer A, Haddad D, Weber M, Gbureck U, Jakob PM, Eulert J, Nöth U (2008) Iron oxide labelling of human mesenchymal stem cells in collagen hydrogels for articular cartilage repair. Biomaterials 29: 1473–1483
Acher P, Popert R, Nichol J, Potters L, Morris S, Beaney R (2006) Permanent prostate brachytherapy: dosimetric results and analysis of a learning curve with a dynamic dose-feedback technique. Int J Radiat Oncol Biol Phys 65: 694–698
Cunningham C, Arai T, Yang P, Mcconnell M, Pauly J, Conolly S (2005) Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med 53: 999–1005
Stuber M, Gilson W, Schär M, Kedziorek D, Hofmann L, Shah S, Vonken E, Bulte J, Kraitchman D (2007) Positive contrast visualization of iron oxide-labeled stem cells using inversion-recovery with ON-resonant water suppression (IRON). Magn Reson Med 58: 1072–1077
Mani V, Briley-Saebo K, Itskovich V, Samber D, Fayad Z (2005) Gradient echo acquisition for superparamagnetic particles with positive contrast (GRASP): Sequence characterization in membrane and glass superparamagnetic iron oxide phantoms at 1.5T and 3T. Magn Reson Med 55: 126–135
Seppenwoolde J, Viergever M, Bakker C (2003) Passive tracking exploiting local signal conservation: the white marker phenomenon. Magn Reson Med 50: 784–790
Overall WR, Pauly JM (2007) Field-encoded SSFP: a new method for positive-contrast visualization of paramagnetic agents. Proc Int Soc Magn Reson Med 15: 579
Dahnke H, Liu W, Herzka D, Frank J, Schaeffter T (2008) Susceptibility gradient mapping (SGM): a new postprocessing method for positive contrast generation applied to superparamagnetic iron oxide particle (SPIO)-labeled cells. Magn Reson Med 60: 595–603
Liu W, Dahnke H, Jordan E, Schaeffter T, Frank J (2008) In vivo MRI using positive-contrast techniques in detection of cells labeled with superparamagnetic iron oxide nanoparticles. NMR Biomed 21: 242–250
Schenck JF (1996) The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys 23: 815–850
Pels K, Labinaz M, Hoffert C, O’Brien ER (1999) Adventitial angiogenesis early after coronary angioplasty: correlation with arterial remodeling. Arterioscler Thromb Vasc Biol 19: 229–238
Dahnke H, Schaeffter T (2005) Limits of detection of SPIO at 3.0 T using T2* relaxometry. Magn Reson Med 53: 1202–1206
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11: 431–441
Gudbjartsson H, Patz S (1995) The Rician distribution of noisy MRI data. Magn Reson Med 34: 910–914
Miller AJ, Joseph PM (1993) The use of power images to perform quantitative analysis on low SNR MR images. Magn Reson Imaging 11: 1051–1056
Mansfield P (1984) Spatial mapping of the chemical shift in NMR. Magn Reson Med 1: 370–386
Bourgeois D, Deslauriers R (1992) Phasing spin-echo-acquired 31P spectroscopic images using complex conjugate data reversal. Magn Reson Med 28: 122–128
Allen JB, Rabiner LR (1977) A unified approach to short-time Fourier analysis. Proc IEEE 65: 1558–1564
Reichenbach JR, Venkatesan R, Yablonskiy DA, Thompson MR, Lai S, Haacke EM (1997) Theory and application of static field inhomogeneity effects in gradient-echo imaging. J Magn Reson Imaging 7: 266–279
Lüdeke KM, Röschmann P, Tischler R (1985) Susceptibility artefacts in NMR imaging. Magn Reson Imaging 3: 329–343
Boulby PA, Rugg-Gunn FJ (2003) T2: the Transverse Relaxation Time. In: Tofts P (eds) Quantitative MRI of the brain. Wiley, Chichester, pp 143–202
Dahnke H, Liu W, Frank JA, Schaeffter T (2007) Suppression of large scale susceptibility artifacts in positive contrast images. Proc Int Soc Magn Reson Med 15: 1228
Vonken E, Schär M, Stuber M (2008) Positive contrast visualization of nitinol devices using susceptibility gradient mapping. Magn Reson Med 60: 588–594
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varma, G., Pedersen, S.F., Taupitz, M. et al. Utilizing different methods for visualizing susceptibility from a single multi-gradient echo dataset. Magn Reson Mater Phy 22, 297–308 (2009). https://doi.org/10.1007/s10334-009-0180-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-009-0180-4