Abstract
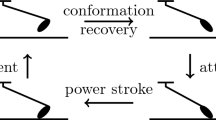
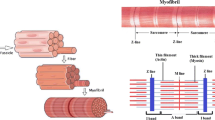
We propose a chemical–mechanical model of myosin heads in sarcomeres, within the classical description of rigid sliding filaments. In our case, myosin heads have two mechanical degrees-of-freedom (dofs)—one of which associated with the so-called power stroke—and two possible chemical states, i.e., bound to an actin site or not. Our major motivations are twofold: (1) to derive a multiscale coupled chemical–mechanical model and (2) to thus account—at the macroscopic scale—for mechanical phenomena that are out of reach for classical muscle models. This model is first written in the form of Langevin stochastic equations, and we are then able to obtain the corresponding Fokker–Planck partial differential equations governing the probability density functions associated with the mechanical dofs and chemical states. This second form is important, as it allows to monitor muscle energetics and also to compare our model with classical ones, such as the Huxley’57 model to which our equations are shown to reduce under two different types of simplifying assumptions. This provides insight and gives a Langevin form for Huxley’57. We then show how we can calibrate our model based on experimental data—taken here for skeletal muscles—and numerical simulations demonstrate the adequacy of the model to represent complex physiological phenomena, in particular the fast isometric transients in which the power stroke is known to have a crucial role, thus circumventing a limitation of many classical models.










Similar content being viewed by others
References
Akalp U, Vernerey FJ (2016) The role of catch-bonds in acto-myosin mechanics and cell mechano-sensitivity. Phys Rev E. https://doi.org/10.1103/PhysRevE.94.012403
Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2014) Molecular biology of the cell, 6h edn. Garland Science, New York
Bestel J, Clément F, Sorine M (2001) A biomechanical model of muscle contraction. In: Niessen W, Viergever M (eds) Lecture Notes in Computer Science, vol 2208. Springer
Brunello E, Reconditi M, Elangovan R, Linari M, Sun Y, Narayanan T, Panine P, Piazzesi G, Irving M, Lombardi V (2007) Skeletal muscle resists stretch by rapid binding of the second motor domain of myosin to actin. PNAS 104(50):20,114–20,119
Brunello E, Caremani M, Melli L, Linari M, Fernandez-Martinez M, Narayanan T, Irving M, Piazzesi G, Lombardi V, Reconditi M (2014) The contributions of filaments and cross-bridges to sarcomere compliance in skeletal muscle. J Physiol 592(17):3881–3899
Caremani M, Melli L, Dolfi M, Lombardi V, Linari M (2015) Force and number of myosin motors during muscle shortening and the coupling with the release of the ATP hydrolysis products. J Physiol 593(15):3313–3332
Caremani M, Pinzauti F, Reconditi M, Piazzesi G, Stienen GJM, Lombardi V, Linari M (2016) Size and speed of the working stroke of cardiac myosin in situ. PNAS 113(13):3675–3680
Caruel M, Truskinovsky L (2018) Physics of muscle contraction. Rep Prog Phys 81:036602
Caruel M, Allain JM, Truskinovsky L (2013) Muscle as a metamaterial operating near a critical point. Phys Rev Lett 110:248103
Caruel M, Chabiniok R, Moireau P, Lecarpentier Y, Chapelle D (2014) Dimensional reductions of a cardiac model for effective validation and calibration. Biomech Model Mechanobiol 13(4):897–914
Chabiniok R, Moireau P, Lesault PF, Rahmouni A, Deux JF, Chapelle D (2012) Estimation of tissue contractility from cardiac cine-MRI using a biomechanical heart model. Biomech Model Mechanobiol 11(5):609–630
Chapelle D, Le Tallec P, Moireau P, Sorine M (2012) Energy-preserving muscle tissue model: formulation and compatible discretizations. J Multiscale Comput Eng 10(2):189–211
Chen YD, Hill TL (1974) Analysis of a simple prototypal muscle model near to and far from equilibrium. Proc Natl Acad Sci USA 71(5):1982–1986
de Tombe PP, Mateja RD, Tachampa K, Mou YA, Farman GP, Irving TC (2010) Myofilament length dependent activation. J Mol Cell Cardiol 48(5):851–858
Dominguez R, Freyzon Y, Trybus KM, Cohen C (1998) Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-power stroke state. Cell 94(5):559–571
Duke T (2000) Cooperativity of myosin molecules through strain-dependent chemistry. Philos T Roy Soc B 355(1396):529–538
Edman KAP, Hwang JC (1977) The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. J Physiol 269:255–272
Eisenberg E, Hill TL (1978) A cross-bridge model of muscle contraction. Prog Biophys Mol Biol 33(1):55–82
Erdmann T, Schwarz U (2012) Stochastic force generation by small ensembles of myosin II motors. Phys Rev Lett 108(18):188101
Ford LE, Huxley AF, Simmons RM (1977) Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol 269(2):441–515
Ford LE, Huxley AF, Simmons RM (1981) The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol 311(1):219–249
Fusi L, Brunello E, Reconditi M, Piazzesi G, Lombardi V (2014) The non-linear elasticity of the muscle sarcomere and the compliance of myosin motors. J Physiol 592(5):1109–1118
Givli S (2010) Towards multi-scale modeling of muscle fibers with sarcomere non-uniformities. J Theor Biol 264(3):882–892
Guérin T, Prost J, Joanny JF (2011) Dynamical behavior of molecular motor assemblies in the rigid and crossbridge models. Eur Phys J E 34(6):60
Guo B, Guilford WA (2006) Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. PNAS 103(26):9844–9849
Hill TL (1974) Theoretical formalism for the sliding filament model of contraction of striated muscle Part I. Prog Biophys Molec Biol 28:267–340
Hill TL (1976) Theoretical formalism for the sliding filament model of contraction of striated muscle part II. Prog Biophys Molec Biol 29:105–159
Hill TL (2004) Free Energy Transduction And Biochemical Cycle kinetics. Dover, Mineola
Hill TL, Chen YD (1974) Further analysis of simple prototypal muscle model near and far from equilibrium. PNAS 71(3):2478–2481
Hill TL, Eisenberg E, Chen YD, Podolsky RJ (1975) Some self-consistent two-state sliding filament models of muscle contraction. Biophys J 15(5):335–372
Howard J (2001) Mechanics of motor proteins and the cytoskeleton. Sinauer Associates Incorporated, Sunderland
Hunter PJ, McCulloch AD, ter Keurs HEDJ (1998) Modelling the mechanical properties of cardiac muscle. Prog Biophys Mol Biol 69:289–331
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Mol Biol 7:258–318
Huxley AF, Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233(5321):533–538
Le Bris C, Lelièvre T (2009) Multiscale modelling of complex fluids: a mathematical initiation. In: Engquist B, Lötstedt P, Runborg O (eds) Multiscale modeling and simulation in science. Springer, Berlin, pp 49–137
Lecarpentier Y, Chemla D, Blanc FX, Pourny JC, Joseph T, Riou B, Coirault C (1998) Mechanics, energetics, and crossbridge kinetics of rabbit diaphragm during congestive heart failure. FASEB J 12(11):981–989
Linari M, Dobbie I, Reconditi M, Koubassova N, Irving M, Piazzesi G, Lombardi V (1998) The stiffness of skeletal muscle in isometric contraction and rigor: the fraction of myosin heads bound to actin. Biophys J 74(5):2459–2473
Linari M, Caremani M, Piperio C, Brandt P, Lombardi V (2007) Stiffness and fraction of myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys J 92(7):2476–2490
Linari M, Piazzesi G, Lombardi V (2009) The effect of myofilament compliance on kinetics of force generation by myosin motors in muscle. Biophys J 96(2):583–592
Linari M, Brunello E, Reconditi M, Fusi L, Caremani M, Narayanan T, Piazzesi G, Lombardi V, Irving M (2015) Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 528(7581):276–279
Lombardi V, Piazzesi G, Linari M (1992) Rapid regeneration of the actin myosin power stroke in contracting muscle. Nature 355(6361):638–641
Lymn RW, Taylor EW (1971) Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10(25):4617–4624. https://doi.org/10.1021/bi00801a004
Marcucci L, Truskinovsky L (2010a) Mechanics of the power stroke in myosin II. Phys Rev E 81(5):051915
Marcucci L, Truskinovsky L (2010b) Muscle contraction: a mechanical perspective. Eur Phys J E 32(4):411–418
Marcucci L, Washio T, Yanagida T (2016) Including thermal fluctuations in actomyosin stable states increases the predicted force per motor and macroscopic efficiency in muscle modelling. PLoS Comput Biol 12(9):e1005083
McMahon TA (1984) Muscles, reflexes, and locomotion. Princeton University Press, Princeton
Mobley BA, Eisenberg BR (1975) Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol 66(1):31–45
Moss RL, Fitzsimons DP (2002) Frank-Starling relationship–long on importance, short on mechanism. Circ Res 90(1):11–13
Niederer SA, Smith NP (2009) The role of the Frank-Starling Law in the transduction of cellular work to whole organ pump function: a computational modeling analysis. PLoS Comput Biol 5:e1000371
Offer G, Ranatunga KW (2016) Reinterpretation of the tension response of muscle to stretches and releases. Biophys J 111(9):2000–2010
Peskin CS (1975) Mathematical aspects of heart physiology. Courant Institute of Mathematical Sciences, NYU
Piazzesi G, Francini F, Linari M, Lombardi V (1992) Tension transients during steady lengthening of tetanized muscle fibres of the frog. J Physiol 445:659–711
Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V (1997) Cross-bridge detachment and attachment following a step stretch imposed on active single frog muscle fibres. J Physiol 498:3–15
Piazzesi G, Lucii L, Lombardi V (2002) The size and the speed of the working stroke of muscle myosin and its dependence on the force. J Physiol 545(1):145–151
Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V (2007) Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131(4):784–795
Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA (1993) Structure of the actin-myosin complex and its implications for muscle contraction. Science 261(5117):58–65
Reconditi M (2006) Recent improvements in small angle x-ray diffraction for the study of muscle physiology. Rep Prog Phys 69(23):2709–2759
Sainte-Marie J, Chapelle D, Cimrman R, Sorine M (2006) Modeling and estimation of the cardiac electromechanical activity. Comput Struct 84(28):1743–1759
Sequeira V, Velden J (2017) The Frank-Starling Law: a jigsaw of titin proportions. Biophys Rev 9(3):259–267
Sheshka R, Truskinovsky L (2014) Power-stroke-driven actomyosin contractility. Phys Rev E 89(1):012708
Smith DA, Mijailovich SM (2008) Towards a unified theory of muscle contraction. 2: predictions with the mean-field approximation. Ann Biomed Eng 36:1353–1371
Smith DA, Geeves MA, Sleep J, Mijailovich SM (2008) Towards a unified theory of muscle contraction. 1: foundations. Ann Biomed Eng 36:1624–1640
Tortora GI, Derrikson B (2009) Principles of anatomy and physiology, 12th edn. Wiley, Hoboken
Veigel C, Molloy JE, Schmitz S, Kendrick-Jones J (2003) Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat Cell Biol 5(11):980–986
Washio T, Sugiura S, Kanada R, Okada JI, Hisada T (2018) Coupling Langevin dynamics with continuum mechanics: exposing the role of sarcomere stretch activation mechanisms to cardiac function. Front Physiol 9:333
Zahalak GI (1981) A distribution-moment approximation for kinetic theories of muscular contraction. Math Biosci 55(1–2):89–114
Zahalak GI (2000) The two-state cross-bridge model of muscle is an asymptotic limit of multi-state models. J Theor Biol 204(1):67–82
Zahalak GI, Ma SP (1990) Muscle activation and contraction: constitutive relations based directly on cross-bridge kinetics. J Biomech Eng 112(1):52–62
Acknowledgements
We would like to warmly thank our colleagues from the Laboratory of Physiology of Firenze University—Vincenzo Lombardi and Marco Linari, in particular—for their invaluable feedback on this work, François Kimmig (Ecole Polytechnique and Inria) for insightful discussions on the thermal equilibrium model and Lev Truskinovsky (ESPCI) for stimulating exchanges on the stochastic model.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A Summary of the main symbols used in the paper
A Summary of the main symbols used in the paper
Symbol | Definition | First occurrence |
|---|---|---|
\( X^t \) | Location of the head tip | p. 4 |
\( Y^t \) | Internal dof of the head (power stroke) | p. 4 |
\( s \) | Position of the nearest actin binding site | p. 4 |
\( \alpha ^t \) | attachment state of the head (0 or 1) | p. 4 |
\({\dot{x}}_{c}\) | Relative velocity of actin filament | p.4 |
\( u_{\alpha } \), \( w_\alpha \), \( u_{e} \) | Energy of a myosin head in state \( \alpha \) (\(w_\alpha (x,y) = u_{\alpha }(y) + u_{e}(x+y)\)) | p. 4 |
\( \eta _x \), \( \eta _y \), \( \eta \) | Drag coefficients | p .4 |
\( D \) | Diffusion coefficient | (2) |
\( P_{\alpha }(s,t) \) | Probability to be attached (\( \alpha =1 \)) or detached (\( \alpha =0 \)) | p. 5, (5) |
\( p(x,y,\alpha ;s,t) \) | Probability function for \((x,y,\alpha )\) (densities in (x, y), discrete in \(\alpha \)) | p. 5, (8) |
\( {\overline{p}}(y;s,t) \) | Effective density in the attached state | (8) |
\( k_{\pm } \) | Attachment (\( k_{+} \)) and detachment (\( k_{-} \)) rates | (4) |
\( f \), \( g \) | Overall attachment (\( f \)) and detachment (\( g \)) functions | |
\(\mu _\alpha \) | Chemical potentials for each state | |
F | Free energy | (24) |
\({\dot{S}}_{prod}\) | Entropy production rate | p. 8 |
\( \tau _{c} \) | Average active tension | (25) |
\( {\overline{J}}_{1}^{0} \) | Flux of the detachment reaction | (26) |
\( \mu _{T} \) | Chemical potential of ATP | p. 8 |
\( p^{th}_\alpha \) | Density functions within the thermal equilibrium hypothesis | (34) |
\( f^{th} \), \( g^{th} \) | Overall attachment and detachment function within the thermal equilibrium hypothesis | (36a), (36b) |
\(T_c\) | Active tension under assumptions on thermal equilibrium and on \(f+g\) | p. 10 |
\(K_c\) | Active stiffness under assumptions on thermal equilibrium and on \(f+g\) | p. 10 |
\({\dot{e}}_{c}\) | Extension rate of sarcomere | p. 10 |
\( \tau _c^{th}\) | Average tension generated by the attached cross-bridges within the thermal equilibrium hypothesis | (46) |
\( \check{P}_{1}^{th}\) | Probability to be attached within the thermal equilibrium hypothesis, in isometric condition | (43) |
\( \check{n}_{1}^{th}\) | Fraction of attached heads within the thermal equilibrium hypothesis, in isometric condition | (44) |
\( \check{\tau }_c^{th}\) | Average tension within the thermal equilibrium hypothesis, in isometric condition | (45) |
\( T_0 \) | Isometric tension | (48) |
\( K_0 \) | Isometric stiffness | (48) |
\( T_{1} \) | Tension reached at the end of a length step | (49) |
\( T_{2} \) | Tension reached after the fast isometric transient following a length step | (50) |
Rights and permissions
About this article
Cite this article
Caruel, M., Moireau, P. & Chapelle, D. Stochastic modeling of chemical–mechanical coupling in striated muscles. Biomech Model Mechanobiol 18, 563–587 (2019). https://doi.org/10.1007/s10237-018-1102-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-018-1102-z