Abstract
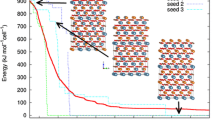
The potential energy hypersurface associated with sodium-potassium alloy clusters is explored via an enhanced genetic algorithm, where two different operators are added to the standard evolutionary procedure. Based on the recent result that the empirical Gupta many-body potential yields reasonable results for clusters with more than seven atoms, we have employed this function in the evaluation of the energies. Agglomerates from seven to the well-established 55-atom structure are studied, and their second-order energy difference and excess energies are calculated. It is found that the most stable alloys (compared to the homonuclear counterparts) are found with the proportion of sodium atoms in the range of 30 to 40%. The experimental propensity of core-shell segregation is successfully predicted by the current approach.






Similar content being viewed by others
References
Rodrigues DDC, Nascimento AM, Duarte HA, Belchior JC (2008) Chem Phys 349:91
Aguado A, López JM (2010) J Chem Phys 133:094302
Jensen P (1999) Rev Mod Phys 71:1695
Baletto F, Ferrando R (2005) Rev Mod Phys 77:371
Chen C, Kang Y, Huo Z, Zhu Z, Huang W, Xin HL, Snyder JD, Li D, Herron JA, Mavrikakis M, Chi M, More KL, Li Y, Markovic NM, Somorjai GA, Yang P, Stamenkovic VR (2014) Science 343:1339
Wilcoxon JP, Provencio PP (2004) J Am Chem Soc 126:6402
Tchaplyguine M, Legendre S, Rosso A, Bradeanu I, Öhrwall G, Canton SE, Andersson T, Mårtensson N, Svensson S, Björneholm O (2009) Phys Rev B 80:033405
Silva MX, Galvão BRL, Belchior JC (2014) Phys Chem Chem Phys 16:8895
Bréchignac C, Cahuzac P, Carlier F, Leygnier J (1989) Chem Phys Lett 164:433
Selby K, Vollmer M, Masui J, Kresin V, de Heer WA, Knight WD (1989) Phys Rev B 40:5417
Selby K, Kresin V, Masui J, Vollmer M, de Heer WA, Scheidemann A, Knight WD (1991) Phys Rev B 43: 4565
Solov’yov IA, Solov’yov AV, Greiner W (2002) Phys Rev A 65:053203
Knight WD, Clemenger K, de Heer WA, Saunders WA (1985) Phys Rev B 31:2539
Knight WD, Clemenger K, de Heer WA, Saunders WA, Chou MY, Cohen ML (1984) Phys Rev Lett 52: 2141
Banerjee A, Ghanty TK, Chakrabarti A (2008) J Phys Chem A 112:12303
Chandrakumar KRS, Ghanty TK, Ghosha SK (2004) J Chem Phys 120:6487
Bonačić-Koutecký V, Fantucci P, Koutecký J (1988) Phys Rev B 37:4369
Kornath A, Ludwig R, Zoermer A (1998) Angew Chem Int Ed 37:1575
Hauser AW, Callegari C, Soldán P, Ernst WE (2008) J Chem Phys 129:044307
González DJ, González LE, Stott MJ (2005) Phys Rev Lett 94:07781
Aguado A, López JM (2011) J Chem Phys 135:134305
Alexandrova AN, Boldyrev AI (2005) J Chem Theory Comput 1:566
Alexandrova AN, Boldyrev AI, Li X, Sarkas HW, Hendricks JH, Arnold ST, Bowen KH (2011) J Chem Phys 134:044322
Li Y, Blaisten-Barojas E, Papaconstantopoulos DA (1998) Phys Rev B 57:15549
Reyes-Nava JA, Garzón IL, Beltrán MR, Michaelian K (2002) Rev Mex Fís 48:450
Gupta RP (1981) Phys Rev B 23:6265
Cleri F, Rosato V (1993) Phys Rev B 48:22
Guimarães FF, Belchior JC, Johnston RL, Roberts C (2002) J Chem Phys 116:8327
Lordeiro RA, Guimarães FF, Belchior JC, Johnston RL (2003) Int J Quantum Chem 95:112
Schlegel HB (1987) Adv Chem Phys 67:249
Zeiri Y (1995) Phys Rev E 51:R2769
Roberts C, Johnston RL, Wilson N (2000) Theoret Chim Acta 104:123
Lai SK, Hsu PJ, Wu KL, Liu WK, Iwamatsu M (2002) J Chem Phys 117:10715
Ferrando R, Jellinek J, Johnston RL (2008) Chem Rev (Washington, D.C.) 108:845
Jellinek J, Krissinel EB (1996) Chem Phys Lett 258:283–292
Acknowledgments
This work was financially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection Brazilian Symposium of Theoretical Chemistry (SBQT2013)
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, M.X., Galvão, B.R.L. & Belchior, J.C. Growth analysis of sodium-potassium alloy clusters from 7 to 55 atoms through a genetic algorithm approach. J Mol Model 20, 2421 (2014). https://doi.org/10.1007/s00894-014-2421-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2421-3