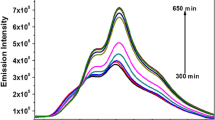
Abstract
The discovery of aggregation inhibitors and the elucidation of their mechanism of action are key in the quest to mitigate the toxic consequences of amyloid formation. We have previously characterized the antiamyloidogenic mechanism of action of sodium phtalocyanine tetrasulfonate ([Na4(H2PcTS)]) on α-Synuclein (αS), demonstrating that specific aromatic interactions are fundamental for the inhibition of amyloid assembly. Here we studied the influence that metal preferential affinity and peripheral substituents may have on the activity of tetrapyrrolic compounds on αS aggregation. For the first time, our laboratory has extended the studies in the field of the bioinorganic chemistry and biophysics to cellular biology, using a well-established cell-based model to study αS aggregation. The interaction scenario described in our work revealed that both N- and C-terminal regions of αS represent binding interfaces for the studied compounds, a behavior that is mainly driven by the presence of negatively or positively charged substituents located at the periphery of the macrocycle. Binding modes of the tetrapyrrole ligands to αS are determined by the planarity and hydrophobicity of the aromatic ring system in the tetrapyrrolic molecule and/or the preferential affinity of the metal ion conjugated at the center of the macrocyclic ring. The different capability of phthalocyanines and meso-tetra (N-methyl-4-pyridyl) porphine tetrachloride ([H2PrTPCl4]) to modulate αS aggregation in vitro was reproduced in cell-based models of αS aggregation, demonstrating unequivocally that the modulation exerted by these compounds on amyloid assembly is a direct consequence of their interaction with the target protein.




Similar content being viewed by others
References
Gandhi S, Wood NW (2010) Genome-wide association studies: the key to unlocking neurodegeneration? Nat Neurosci 13:789–794. https://doi.org/10.1038/nn.2584
de Lau LML, Breteler MMB (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535. https://doi.org/10.1016/S1474-4422(06)70471-9
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4:600–609. https://doi.org/10.1038/ncpneuro0924
Spillantini MG, Crowther RA, Jakes R et al (1998) Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 251:205–208. https://doi.org/10.1016/S0304-3940(98)00504-7
Papapetropoulos S, Adi N, Ellul J et al (2007) A prospective study of familial versus sporadic Parkinson’s disease. Neurodegener Dis 4:424–427. https://doi.org/10.1159/000107702
Dawson TM, Dawson VL (2003) Rare genetic mutations shed light on the pathogenesis of Parkinson disease. J Clin Invest 111:145–151. https://doi.org/10.1172/JCI17575
McCann H, Stevens CH, Cartwright H, Halliday GM (2014) α-Synucleinopathy phenotypes. Parkinsonism Relat Disord 20(Suppl 1):S62–S67. https://doi.org/10.1016/S1353-8020(13)70017-8
Spillantini MG, Crowther RA, Jakes R et al (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA 95:6469–6473. https://doi.org/10.1073/pnas.95.11.6469
Volles MJ, Lansbury PT (2003) Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42:7871–7878. https://doi.org/10.1021/bi030086j
Cookson MR, van der Brug M (2008) Cell systems and the toxic mechanism(s) of alpha-synuclein. Exp Neurol 209:5–11. https://doi.org/10.1016/j.expneurol.2007.05.022
Eisbach SE, Outeiro TF (2013) Alpha-synuclein and intracellular trafficking: impact on the spreading of Parkinson’s disease pathology. J Mol Med 91:693–703. https://doi.org/10.1007/s00109-013-1038-9
Roberts HL, Brown DR (2015) Seeking a mechanism for the toxicity of oligomeric α-synuclein. Biomolecules 5:282–305. https://doi.org/10.3390/biom5020282
Winner B, Jappelli R, Maji SK et al (2011) In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA 108:4194–4199. https://doi.org/10.1073/pnas.1100976108
Karpinar DP, Balija MBG, Kügler S et al (2009) Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J 28:3256–3268. https://doi.org/10.1038/emboj.2009.257
Lázaro DF, Rodrigues EF, Langohr R et al (2014) Systematic comparison of the effects of alpha-synuclein mutations on its oligomerization and aggregation. PLoS Genet 10:e1004741. https://doi.org/10.1371/journal.pgen.1004741
Pokrzywa M, Pawełek K, Kucia WE et al (2017) Effects of small-molecule amyloid modulators on a Drosophila model of Parkinson’s disease. PLoS One 12:e0184117. https://doi.org/10.1371/journal.pone.0184117
Kurnik M, Sahin C, Andersen CB et al (2018) Potent α-synuclein aggregation inhibitors, identified by high-throughput screening, mainly target the monomeric state. Cell Chem Biol 25:1389–1402.e9. https://doi.org/10.1016/j.chembiol.2018.08.005
Tonda-Turo C, Herva M, Chiono V et al (2018) Influence of drug-carrier polymers on alpha-synucleinopathies: a neglected aspect in new therapies development. Biomed Res Int 2018:4518060. https://doi.org/10.1155/2018/4518060
Trigo-Damas I, Del Rey NL-G, Blesa J (2018) Novel models for Parkinson’s disease and their impact on future drug discovery. Expert Opin Drug Discov 13:229–239. https://doi.org/10.1080/17460441.2018.1428556
Maroteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8:2804–2815
Burré J, Sharma M, Südhof TC (2014) α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA 111:E4274–E4283. https://doi.org/10.1073/pnas.1416598111
Stefanis L (2012) α-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med 2:a009399. https://doi.org/10.1101/cshperspect.a009399
Sidhu A, Wersinger C, Vernier P (2004) Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J 18:637–647. https://doi.org/10.1096/fj.03-1112rev
Yavich L, Tanila H, Vepsäläinen S, Jäkälä P (2004) Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci 24:11165–11170. https://doi.org/10.1523/JNEUROSCI.2559-04.2004
Breydo L, Wu JW, Uversky VN (2012) Α-synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta 1822:261–285. https://doi.org/10.1016/j.bbadis.2011.10.002
Dedmon MM, Lindorff-Larsen K, Christodoulou J et al (2005) Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J Am Chem Soc 127:476–477. https://doi.org/10.1021/ja044834j
Bertoncini CW, Jung Y-S, Fernandez CO et al (2005) Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci USA 102:1430–1435. https://doi.org/10.1073/pnas.0407146102
Cho M-K, Nodet G, Kim H-Y et al (2009) Structural characterization of alpha-synuclein in an aggregation prone state. Protein Sci 18:1840–1846. https://doi.org/10.1002/pro.194
Chiti F, Dobson CM (2017) Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem 86:27–68. https://doi.org/10.1146/annurev-biochem-061516-045115
Moriarty GM, Janowska MK, Kang L, Baum J (2013) Exploring the accessible conformations of N-terminal acetylated α-synuclein. FEBS Lett 587:1128–1138. https://doi.org/10.1016/j.febslet.2013.02.049
Kang L, Moriarty GM, Woods LA et al (2012) N-terminal acetylation of α-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci 21:911–917. https://doi.org/10.1002/pro.2088
Liu T, Bitan G (2012) Modulating self-assembly of amyloidogenic proteins as a therapeutic approach for neurodegenerative diseases: strategies and mechanisms. ChemMedChem 7:359–374. https://doi.org/10.1002/cmdc.201100585
Villemagne VL, Doré V, Bourgeat P et al (2017) Aβ-amyloid and Tau Imaging in Dementia. Semin Nucl Med 47:75–88. https://doi.org/10.1053/j.semnuclmed.2016.09.006
Arja K, Sjölander D, Åslund A et al (2013) Enhanced fluorescent assignment of protein aggregates by an oligothiophene-porphyrin-based amyloid ligand. Macromol Rapid Commun 34:723–730. https://doi.org/10.1002/marc.201200817
Lamberto GR, Binolfi A, Orcellet ML et al (2009) Structural and mechanistic basis behind the inhibitory interaction of PcTS on alpha-synuclein amyloid fibril formation. Proc Natl Acad Sci USA 106:21057–21062. https://doi.org/10.1073/pnas.0902603106
Valiente-Gabioud AA, Riedel D, Outeiro TF et al (2018) Binding modes of phthalocyanines to amyloid β peptide and their effects on amyloid fibril formation. Biophys J 114:1036–1045. https://doi.org/10.1016/j.bpj.2018.01.003
Lamberto GR, Torres-Monserrat V, Bertoncini CW et al (2011) Toward the discovery of effective polycyclic inhibitors of alpha-synuclein amyloid assembly. J Biol Chem 286:32036–32044. https://doi.org/10.1074/jbc.M111.242958
Bulic B, Pickhardt M, Khlistunova I et al (2007) Rhodanine-based tau aggregation inhibitors in cell models of tauopathy. Angew Chem Int Ed Engl 46:9215–9219. https://doi.org/10.1002/anie.200704051
Schenk D, Basi GS, Pangalos MN (2012) Treatment strategies targeting amyloid β-protein. Cold Spring Harb Perspect Med 2:a006387. https://doi.org/10.1101/cshperspect.a006387
Masuda M, Suzuki N, Taniguchi S et al (2006) Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry 45:6085–6094. https://doi.org/10.1021/bi0600749
Caughey B, Caughey WS, Kocisko DA et al (2006) Prions and transmissible spongiform encephalopathy (TSE) chemotherapeutics: a common mechanism for anti-TSE compounds? Acc Chem Res 39:646–653. https://doi.org/10.1021/ar050068p
Ehrnhoefer DE, Bieschke J, Boeddrich A et al (2008) EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol 15:558–566. https://doi.org/10.1038/nsmb.1437
Caughey WS, Priola SA, Kocisko DA et al (2007) Cyclic tetrapyrrole sulfonation, metals, and oligomerization in antiprion activity. Antimicrob Agents Chemother 51:3887–3894. https://doi.org/10.1128/AAC.01599-06
Wagner J, Ryazanov S, Leonov A et al (2013) Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol 125:795–813. https://doi.org/10.1007/s00401-013-1114-9
Levin J, Schmidt F, Boehm C et al (2014) The oligomer modulator anle138b inhibits disease progression in a Parkinson mouse model even with treatment started after disease onset. Acta Neuropathol 127:779–780. https://doi.org/10.1007/s00401-014-1265-3
Scherzer-Attali R, Shaltiel-Karyo R, Adalist YH et al (2012) Generic inhibition of amyloidogenic proteins by two naphthoquinone-tryptophan hybrid molecules. Proteins 80:1962–1973. https://doi.org/10.1002/prot.24080
Frydman-Marom A, Shaltiel-Karyo R, Moshe S, Gazit E (2011) The generic amyloid formation inhibition effect of a designed small aromatic β-breaking peptide. Amyloid 18:119–127. https://doi.org/10.3109/13506129.2011.582902
Frydman-Marom A, Rechter M, Shefler I et al (2009) Cognitive-performance recovery of Alzheimer’s disease model mice by modulation of early soluble amyloidal assemblies. Angew Chem Int Ed Engl 48:1981–1986. https://doi.org/10.1002/anie.200802123
González-Lizárraga F, Socías SB, Ávila CL et al (2017) Repurposing doxycycline for synucleinopathies: remodelling of α-synuclein oligomers towards non-toxic parallel beta-sheet structured species. Sci Rep 7:41755. https://doi.org/10.1038/srep41755
Pujols J, Peña-Díaz S, Lázaro DF et al (2018) Small molecule inhibits α-synuclein aggregation, disrupts amyloid fibrils, and prevents degeneration of dopaminergic neurons. Proc Natl Acad Sci USA 115:10481–10486. https://doi.org/10.1073/pnas.1804198115
Valdinocci D, Grant GD, Dickson TC, Pountney DL (2018) Epothilone D inhibits microglia-mediated spread of alpha-synuclein aggregates. Mol Cell Neurosci 89:80–94. https://doi.org/10.1016/j.mcn.2018.04.006
Schwab K, Frahm S, Horsley D et al (2017) A protein aggregation inhibitor, leuco-methylthioninium bis(hydromethanesulfonate), decreases α-synuclein inclusions in a transgenic mouse model of synucleinopathy. Front Mol Neurosci 10:447. https://doi.org/10.3389/fnmol.2017.00447
Palazzi L, Bruzzone E, Bisello G et al (2018) Oleuropein aglycone stabilizes the monomeric α-synuclein and favours the growth of non-toxic aggregates. Sci Rep 8:8337. https://doi.org/10.1038/s41598-018-26645-5
Jha NN, Kumar R, Panigrahi R et al (2017) Comparison of α-synuclein fibril inhibition by four different amyloid inhibitors. ACS Chem Neurosci 8:2722–2733. https://doi.org/10.1021/acschemneuro.7b00261
Reiner AM, Schmidt F, Ryazanov S et al (2018) Photophysics of diphenyl-pyrazole compounds in solutions and α-synuclein aggregates. Biochim Biophys Acta Gen Subj 1862:800–807. https://doi.org/10.1016/j.bbagen.2017.12.007
Valiente-Gabioud AA, Miotto MC, Chesta ME et al (2016) Phthalocyanines as molecular scaffolds to block disease-associated protein aggregation. Acc Chem Res 49:801–808. https://doi.org/10.1021/acs.accounts.5b00507
Fonseca-Ornelas L, Eisbach SE, Paulat M et al (2014) Small molecule-mediated stabilization of vesicle-associated helical α-synuclein inhibits pathogenic misfolding and aggregation. Nat Commun 5:5857. https://doi.org/10.1038/ncomms6857
Akoury E, Gajda M, Pickhardt M et al (2013) Inhibition of tau filament formation by conformational modulation. J Am Chem Soc 135:2853–2862. https://doi.org/10.1021/ja312471h
Chakraborty R, Sahoo S, Halder N et al (2019) Conformational-switch based strategy triggered by [18] π heteroannulenes toward reduction of alpha synuclein oligomer toxicity. ACS Chem Neurosci 10:573–587. https://doi.org/10.1021/acschemneuro.8b00436
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26:267–298. https://doi.org/10.1146/annurev.neuro.26.010302.081142
Caughey WS, Raymond LD, Horiuchi M, Caughey B (1998) Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc Natl Acad Sci USA 95:12117–12122. https://doi.org/10.1073/pnas.95.21.12117
Priola SA, Raines A, Caughey WS (2000) Porphyrin and phthalocyanine antiscrapie compounds. Science 287:1503–1506
Johnson M, Geeves MA, Mulvihill DP (2013) Production of amino-terminally acetylated recombinant proteins in E. coli. Methods Mol Biol 981:193–200. https://doi.org/10.1007/978-1-62703-305-3_15
Hoyer W, Cherny D, Subramaniam V, Jovin TM (2004) Impact of the acidic C-terminal region comprising amino acids 109-140 on alpha-synuclein aggregation in vitro. Biochemistry 43:16233–16242. https://doi.org/10.1021/bi048453u
Cavanagh J, Fairbrother W, Palmer A III, Skelton N (1995) Protein NMR spectroscopy: principles and practice. Academic Press. ISBN: 9780080515298
Valiente-Gabioud AA, Torres-Monserrat V, Molina-Rubino L et al (2012) Structural basis behind the interaction of Zn2+ with the protein α-synuclein and the Aβ peptide: a comparative analysis. J Inorg Biochem 117:334–341. https://doi.org/10.1016/j.jinorgbio.2012.06.011
Acknowledgements
C.O.F. thanks Universidad Nacional de Rosario (UNR) and ANPCyT- FONCyT (PICT 2014-3704 and PICT 2017-4665) for financial support. C.O.F. and C.G. thank the Max Planck Society (P10390) for support. C.O.F thanks Dietmar Riedel and Gudrum Heim for helpful assistance during the transmission electron microscopy measurements. N.G. and I.G. thanks UNR for fellowships. F.B. thanks CONICET for fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González, N., Gentile, I., Garro, H.A. et al. Metal coordination and peripheral substitution modulate the activity of cyclic tetrapyrroles on αS aggregation: a structural and cell-based study. J Biol Inorg Chem 24, 1269–1278 (2019). https://doi.org/10.1007/s00775-019-01711-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01711-z