Abstract
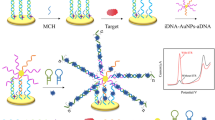
A signal-on aptasensor is described for the voltammetric determination of kanamycin (KANA). Au@Pt core-shell nanoparticles with large surface and good electrical conductivity were synthetized and act as both a conductive material and as the carrier for complementary strands (CS2) and thionine (TH). In the presence of KANA, the electrochemical response of TH changes due to hybridization between CS1 immobilized on the electrode and the Au@Pt-CS2/TH system. The peak current increases linearly with the logarithm of the KANA concentration in the range from 1 pM to 1 μM, and the limit of detection is 0.16 pM. The sensor was characterized in terms of selectivity, reproducibility and stability, and satisfactory results were obtained. It was also utilized for the determination of KANA in (spiked) chicken samples. The recoveries (95.8–103.2%) demonstrate the potential of the method for KANA detection in real samples.

A signal-on aptasensor for kanamycin (KANA) was developed by using Au@Pt core-shell nanoparticles as nanocarrier for probe aptamer and as a sensing probe.






Similar content being viewed by others
References
Ramezani M, Danesh NM, Lavaee P, Abnous K, Taghdisi SM (2016) A selective and sensitive fluorescent aptasensor for detection of kanamycin based on catalytic recycling activity of exonuclease III and gold nanoparticles. Sens Actuator B-Chem 222:1–7
Sharma A, Istamboulie G, Hayat A, Catanante G, Bhand S, Marty JL (2017) Disposable and portable aptamer functionalized impedimetric sensor or detection of kanamycin residue in milk sample. Sens Actuator B-Chem 45:507–515
Song H, Kang T, Li N, Lu L, Cheng S (2016) Highly sensitive voltammetric determination of kanamycin based on aptamer sensor for signal amplification. Anal Methods 8:3366–3372
Robati RY, Arab A, Ramezani M, Langroodi FA, Abnous K, Taghdisi SM (2016) Aptasensors for quantitative detection of kanamycin. Biosens Bioelectron 82:162–172
Guo W, Sun N, Qin X, Pei M, Wang L (2015) A novel electrochemical aptasensor for ultrasensitive detection of kanamycin based on MWCNTs-HMIMPF6 and nanoporous PtTi alloy. Biosens Bioelectron 74:691–697
Wang C, Chen D, Wang Q, Tan R (2017) Kanamycin detection based on the catalytic ability enhancement of gold nanoparticles. Biosens Bioelectron 91:262–267
Tsuji K, Robertson JH (1970) Gas-liquid chromatographic determination of amino-glycoside antibiotics: kanamycin and Paromomycin. Anal Chem 42:1661–1663
Ishii R, Horie M, Chan W, Macneil J (2008) Multi-residue quantitation of aminoglycoside antibiotics in kidney and meat by liquid chromatography with tandem mass spectrometry. Food Addit Contam 25:1509–1519
Arsand JB, Jank L, Martins MT, Hoff RB, Barreto F, Pizzolato TM (2016) Determination of aminoglycoside residues in milk and muscle based on a simple and fast extraction procedure followed by liquid chromatography coupled to tandem mass spectrometry and time of flight mass spectrometry. Talanta 154:38–45
Sun Y, Li D, He S, Liu P, Hu Q, Cao Y (2013) Determination and dynamics of kanamycin a residue in soil by HPLC with SPE and precolumn derivatization. Int J Envior An Ch 93:472–481
Mays DL, Apeldoorn RJV, Lauback RG (1976) High-performance liquid chromatographic determination of kanamycin. J Chromatogr A 120:93–102
Gal'Vidis IA, Burkin MA (2010) Monoclonal antibody based enzyme-linked immunosorbent assay for aminoglycoside antibiotic kanamycin in foodstuff. Bioorg Khim 36:789–796
Fei X, Jing L, Zhou J, Liu M, Liu Y, Wang J (2016) A visual gel-enzyme-linked immunosorbent assay for simultaneous detection of gentamicin and kanamycin in milk. Chinese J Anal Chem 43:881–885
Su P, Chen X, He Z, Yang Y (2017) Preparation of polyclonal antibody and development of a biotin-streptavidin-based ELISA method for detecting kanamycin in milk and honey. Chinese J Anal Chem 33:1–6
Li F, Wang X, Sun X, Guo Y (2018) Multiplex electrochemical aptasensor for detecting multiple antibiotics residues based on carbon fiber and mesoporous carbon-gold nanoparticles. Sens Actuator B-Chem 265:217–226
Arya SK, Zhurauski P, Jolly P, M Batistuti R, Mulato M, Estrela P (2018) Capacitive aptasensor based on interdigitated electrode for breast cancer detection in undiluted human serum. Biosens Bioelectron 102:106–112
Tabrizi MA, Shamsipu M, Sherkatkhameneh N (2017) Flow injection amperometric sandwich-type electrochemical aptasensor for the determination of adenocarcinoma gastric cancer cell using aptamer-au@ag nanoparticles as labeled aptamer. Electrochim Acta 246:1147–1154
Huang KJ, Liu YJ, Zhang JZ (2015) Aptamer-based electrochemical assay of 17β-estradiol using a glassy carbon electrode modified with copper sulfide nanosheets and gold nanoparticles, and applying enzyme-based signal amplification. Microchim Acta 182:409–417
Xu Q, Wang G, Zhang M, Xu G, Lin J, Luo X (2018) Aptamer based label free thrombin assay based on the use of silver nanoparticles incorporated into self-polymerized dopamine. Microchim Acta 185:253–260
Roushani M, Shahdost-Fard F (2018) Impedimetric detection of cocaine by using an aptamer attached to a screen printed electrode modified with a dendrimer/silver nanoparticle nanocomposite. Microchim Acta 185:214–222
Liu D, Lu X, Yang Y, Zhai Y, Zhang J, Li L (2018) A novel fluorescent aptasensor for the highly sensitive and selective detection of cardiac troponin I based on a graphene oxide platform. Anal Bioanal Chem 410:4285–4291
Yin J, Guo W, Qin X, Zhao J, Pei M, Ding F (2017) A sensitive electrochemical aptasensor for highly specific detection of streptomycin based on the porous carbon nanorods and multifunctional graphene nanocomposites for signal amplification. Sens Actuator B-Chem 241:151–159
Wang L, Meng Y, Zhang Y, Zhang C, Xie Q (2017) Photoelectrochemical aptasensing of thrombin based on multilayered gold nanoparticle/graphene-TiO2 and enzyme functionalized graphene oxide nanocomposites. Electrochim Acta 249:243–252
Wu Y, Zou L, Lei S, Yu Q, Ye B (2017) Highly sensitive electrochemical thrombin aptasensor based on peptideenhanced electrocatalysis of hemin/G-quadruplex and nanocomposite as nanocarrier. Biosens Bioelectron 97:317–324
Yuan Y, Yuan R, Chai Y, Zhuo Y, Bai L, Liao Y (2010) A signal-on electrochemical probe-label-free aptasensor using gold–platinum alloy and stearic acid as enhancers. Biosens Bioelectron 26:881–885
Song P, Mei L, Wang A, Fang K, Feng J (2016) One-pot surfactant-free synthesis of porous PtAu alloyed nanoflowers with enhanced electrocatalytic activity for ethanol oxidation and oxygen reduction reactions. Int J Hydrogen Energ 41:1645–1653
Dutta A, Ouyang J (2014) Enhanced electrocatalytic performance on polymer-stabilized graphene decorated with alloy nanoparticles for ethanol oxidation reaction in alkaline media. Appl Catal B-Environ 158-159:119–128
Zan X, Fang Z, Wu J, Xiao F, Huo F, Duan H (2013) Freestanding graphene paper decorated with 2D-assembly of au@Pt core shellnanoparticles as flexible biosensors to monitor live cell secretion of nitric oxide. Biosens Bioelectron 49:71–78
He BS, Yan SS (2018) Electrochemical aptasensor based on aptamer complimentary strand conjugate and thionine for sensitive detection of tetracycline with multiwalled carbon nanotubes and gold nanoparticles amplification. Ana Methods 10:783–790
Qin X, Yin Y, Yu H, Guo W, Pei M (2016) A novel signal amplification strategy of an electrochemical aptasensor for kanamycin, based on thionine functionalized graphene and hierarchical nanoporous PtCu. Biosens Bioelectron 77:752–758
Li F, Wang X, Sun X, Guo Y (2017) An aptasensor with dsDNA for rapid and highly sensitive detection of kanamycin in milk. RSC Adv 7:38981–38988
Li F, Wang X, Sun X, Guo Y, Zhao W (2018) A dual-signal amplification strategy for kanamycin based on ordered mesoporous carbon-chitosan/gold nanoparticles-streptavidin and ferrocene labelled DNA. Anal Chim Acta 1033:185–192
Li F, Guo Y, Sun X, Wang X (2014) Aptasensor based on thionine, graphene–polyaniline composite film, and gold nanoparticles for kanamycin detection. Eur Food Res Technol 239:227–236
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 61301037), the Henan Science and Technology Cooperation Project (Grant No. 172106000014), the Cultivation Plan for Young Core Teachers in Universities of Henan Province (No. 2017GGJS072), the Youth Backbone Teacher Training Program of Henan University of Technology (No. 21420004), and the Master's Degree Thesis Cultivation Project of Henan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 814 kb)
Rights and permissions
About this article
Cite this article
He, B., Yan, S. Voltammetric kanamycin aptasensor based on the use of thionine incorporated into Au@Pt core-shell nanoparticles. Microchim Acta 186, 77 (2019). https://doi.org/10.1007/s00604-018-3188-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3188-5