Abstract
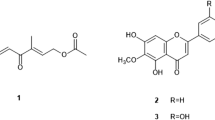
Neglected tropical diseases hinder social and economic growth in many tropical areas. We report leishmanicidal activities of isoflavanquinones and antifungal potentials of fractions from methanol root extract of Abrus precatorius. Agar dilution method was used to determine antifungal properties while 96-well dilution protocol was adopted for antileishmanial activities. Ethyl acetate fraction of A. precatorius root extract showed moderate activity against M. canis and F. solani with percentage inhibition of 42.5% and 55.0% respectively while other fractions were inactive. Crude extract, n-hexane, and ethyl acetate fractions demonstrated potent antileishmanial activities with IC50 values of 22.20 ± 0.540 μg/mL, 19.35 ± 0.670 μg/mL, and 6.32 ± 0.001 μg/mL respectively, against L. major. The ethyl acetate fraction, which was the most active fraction, was subjected to successive column chromatography followed by preparative recycling HPLC in order to isolate the active constituents. Structures were established by HR-ESIMS, 1D and 2D NMR (1H, 13C, COSY, NOESY, HMBC, HSQC) spectroscopy. Two compounds were obtained and identified as isoflavanquinones: abruquinone A (1) and abruquinone B (2). Compounds 1 and 2 demonstrated significant (p < 0.05) antileishmanial activity against L. major (IC50 6.35 ± 0.005 μg/mL and 6.32 ± 0.008 μg/mL respectively) and L. tropica (IC50 6.29 ± 0.015 μg/mL and 6.31 ± 0.005 μg/mL, respectively). This appears to be the first report of antileishmanial activity of compounds 1 and 2 from the genus Abrus against cutaneous leishmaniasis and validates the use of A. precatorius in treating dermatology diseases.


Similar content being viewed by others
References
Advait SN, Shilpi K, Arun BK, Frantisek S, Andriy B, Casey JM, Valentina M (2014) Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem Rev 114:11305–11347
Alvar J, Ve'lez I, Bern C, Herrero M, Desjeux P, Cano J, den Boe M (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7(5):e35671
Androula P, Helena CM (2010) Leishmaniasis, an emerging infection in travelers. Int J Inf Secur 14:e1032–e1039
Aziz AR, Volodymyr S, Surendra KJ, Babu LT, Shabana IK, Melissa RJ, Ilias M (2011) Antiparasitic and antimicrobial isoflavanquinones from Abrus schimperi. Nat Prod Commun 6(11):1645–1650
Bhardwaj D, Bisht M, Mehta C (1980) Flavonoids from Abrus precatorius. Phytochemistry 19:2040–2041
Choi Y, Hussain R, Pezzuto J, Kinghorn A, Morton J (1989) Abrusosides A-D, four novel sweet-tasting triterpene glycosides from the leaves of Abrus precatorius. J Nat 52:1118–1127
Choudhary M, Dur-e-Shahwar PZ, Jabbar A, Ali I, Atta-ur-Rahman (1995) Antifungal steroidal lactones from Withania coagulance. Phytochemistry 40(4):1243–1246
Chutima L, Sinenart S, Prasat K (2004) Antitubercular and antiplasmodial constituents of Abrus precatorius. Planta Med 70:276–278
Gadadhar SS, Karande AA (2013) Abrin immunotoxin: targeted cytotoxicity and intracellular trafficking pathway. PLoS One 8(3):e58304
Gathirwa J, Rukunga G, Mwitari P, Mwikwabe N, Kimani C, Muthaura C, Omar S (2011) Traditional herbal antimalarial therapy in Kilifi District, Kenya. J Ethnopharmacol 134:434–442
Ghosal S, Dutta S (1971) Alkaloids of Abrus precatorius. Phytochemistry 10:195–198
Holetz FB, Perrini GL, Sanches NR, Coritez DAG, Nakamura CV, Filho BPD (2002) Screening of some plants used in the Brazilian Folk Medicine for the treatment of Infectious Diseases. Mem I Oswaldo Cruz 97:7
Kalpesh M, Mehta M, Mendpara N, Garmit S, Shah G (2010) Determination of antibacterial activity of MIC of crude extract of Abrus precatorius L. Bitotechnol Adv 10:2
Kerry O, Deanna AS, Annette F, Dora M, Michael GR, Mary EB, David MG (2008) Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani dpecies complex. J Clin Microbiol 46(8):2477–2490
Kishur SC, Ramakant S, Pradeep SP, Vidyadhish AK (2012) Pharmacological activities of Abrus precatorius Linn – a review. Int J Ayurvedic Herb Med 2(2):336–348
Kuo SC, Chen SC, Chen LH, Wu JB, Wang JP, Teng CM (1995) Potent antiplatelet, anti-inflammatory and antiallergic isoflavanquinones from the roots of Abrus precatorius. Planta Med 61(4):307–312
Lubna M, Syed AS, Rashida A, Syed GS, Rahil A (2018) Antibacterial and antifungal activities of the polyphenolic fractions isolated from the seed coat of Abrus precatorius and Caesalpinia crista. Nat Prod Res 32(23):2835–2839
Lunder M (1992) Is Microsporum canis infection about to become a serious dermatological problem? Dermatology 184:87–89
Max G, Mark H, Wiliam E, Thomas H, John SL, Elizabeth RS, Richard JS (2013) Review: drug discovery algorithm for cutaneous leishmaniasis. Am J Trop Med Hyg 88(2):216–221
Menan H, Banzouzi J, Hocquette A, Pelissier Y, Blache Y, Kone M, Valentin A (2006) Antiplasmodial activity and cytotoxicity of plants used in West Africa traditional medicine for the treatment of malaria. J Ethnopharmacol 105:131–136
Messori A, Fadda V, Maratea D, Trippoli S, Marinaj C (2013) Nephrotoxicity of different formulations of amphotericin B: summarizing evidence by network meta-analysis. Clin Infect Dis 57(12):1783–1784
Michael S, Michael AK, Richard BB (1985) Pentamidine: a review. Clin Infect Dis 7(5):625–634
Reithinger R, Dujardin J, Louzir H, Pirmez C, Alexander B, Brooker S, Lockwood D (2007) Cutaneous leishmaniasis. Clin Dermatol 25:203–211
Song C, Hu Z (1998) Abruquinone A, B, D, E, F, and G from the root of Abrus precatorius. Acta Bot Sin 40:734–739
Xiao Z, Wang F, Sun A, Li C, Huang C, Zhang S (2012) A new triterpenoid saponin from Abrus precatorius Linn. Molecules 17:295–302
Yoshie H, Melanie R, Samad NE, Stefanie Z, Tsholofelo M, Dashnie N, Mathias H (2013) Antiprotozoal isoflavanquinones from Abrus precatorius ssp. Africanus. Planta Med 79:492–498
Acknowledgment
EEO acknowledges the World Academy of Sciences for the advancement of Science in Developing Countries (TWAS), Trieste, Italy, for ICCBS-TWAS Fellowship (2018) at the H.E.J. Research Institute of Chemistry, ICCBS, University of Karachi, Karachi, Pakistan.
Funding
This research work was fully funded by the International Centre for Chemical and Biological Sciences, ICCBS, University of Karachi, Karachi, Pakistan and The World Academy of Science (TWAS) Trieste, Italy, for the advancement of Science in Developing Countries.
Author information
Authors and Affiliations
Contributions
EEO conceptualized and designed the research work; EEO carried out the assays, analyzed data, and wrote the article manuscript, MSA elucidated the chemical structures; MSA, ORO, and FDO proofread the article manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not required
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okoro, E.E., Ahmad, M.S., Osoniyi, O.R. et al. Antifungal and antileishmanial activities of fractions and isolated isoflavanquinones from the roots of Abrus precatorius. Comp Clin Pathol 29, 391–396 (2020). https://doi.org/10.1007/s00580-019-03073-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-03073-z