Abstract
Plant phenological shifts (e.g., earlier flowering dates) are known consequences of climate change that may alter ecosystem functioning, productivity, and ecological interactions across trophic levels. Temperate, subalpine, and alpine regions have largely experienced advancement of spring phenology with climate warming, but the effects of climate change in warm, humid regions and on autumn phenology are less well understood. In this study, nearly 10,000 digitized herbarium specimen records were used to examine the phenological sensitivities of fall- and spring-flowering asteraceous plants to temperature and precipitation in the US Southeastern Coastal Plain. Climate data reveal warming trends in this already warm climate, and spring- and fall-flowering species responded differently to this change. Spring-flowering species flowered earlier at a rate of 1.8–2.3 days per 1 °C increase in spring temperature, showing remarkable congruence with studies of northern temperate species. Fall-flowering species flowered slightly earlier with warmer spring temperatures, but flowering was significantly later with warmer summer temperatures at a rate of 0.8–1.2 days per 1 °C. Spring-flowering species exhibited slightly later flowering times with increased spring precipitation. Fall phenology was less clearly influenced by precipitation. These results suggest that even warm, humid regions may experience phenological shifts and thus be susceptible to potentially detrimental effects such as plant-pollinator asynchrony.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in the timing of species’ life history events—“phenological shifts”—are closely linked to changes in climate and can produce cascading effects across ecosystems, altering ecosystem functioning (Parmesan 2006; Calinger et al. 2013), productivity (Richardson et al. 2010), and ecological interactions such as those between plants and pollinators (Kharouba and Vellend 2015; Forrest 2015) or plants and migratory birds (Both et al. 2006). Despite myriad studies since the turn of the century investigating the effects of climate change on plant phenology (i.e., “phenological sensitivity”) using observational data (e.g., Fitter and Fitter 2002; Ellwood et al. 2014; Tansey et al. 2017), herbarium specimens (e.g., Primack et al. 2004; Lavoie and Lachance 2006; Munson and Long 2016), experiments (e.g., Price and Waser 1998; Pan et al. 2017; Posledovich et al. 2017), and combinations of data sources (e.g., Miller-Rushing et al. 2006; Panchen et al. 2012), significant gaps in our understanding of these phenomena and their potential consequences remain (Willis et al. 2017). Notably, the phenological sensitivity of plants to climate change in warm, humid temperate to subtropical regions, as well as the effects of climate change on autumn phenology, remain poorly understood (Pau et al. 2011; Willis et al. 2017; but see Von Holle et al. 2010; Park and Schwartz 2015; Gallinat et al. 2015).
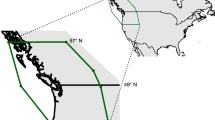
Many studies have discovered negative relationships between temperature and phenological events such as flowering and leaf-out; that is, plants flower or leaf out earlier with increased temperatures in the 2–3 months preceding the phenological event (Sparks et al. 2006; Willis et al. 2017). However, our understanding of these relationships has largely relied upon studies in temperate, boreal, alpine, or subalpine climates such as the Northeastern USA and north-central Europe (Pau et al. 2011; Willis et al. 2017), though some efforts have focused on Mediterranean climates in Spain (Gordo and Sanz 2010) and California (Cleland et al. 2006), subtropical China and India (Hart et al. 2014; Gaira et al. 2014; Chen et al. 2017), coastal Australia (Rumpff et al. 2010), and xeric regions in the western USA (Neil et al. 2010; Munson and Long 2016). This study examines plant phenological sensitivity to temperature and precipitation change in the US Southeastern Coastal Plain (SECP; Fig. 1).
US Southeastern Coastal Plain region selected for sampling of herbarium specimen records (outlined in black). Note the relatively flat topography. Although it is not generally considered within the SECP, south Florida was included to maximize sample size. The northernmost regions of the SECP, including Virginia, Maryland, New Jersey, New York, Connecticut, Rhode Island, and Massachusetts were excluded to reduce the effect of latitude on statistical results. Map created by DEMIS BV and made available via https://commons.wikimedia.org/wiki/File:Map_of_USA_topological.png
The phenological effects of climate change in warm, humid yet temperate climates may provide unique insights into mechanisms of phenological change. Phenological sensitivities of organisms in cooler regions may be constrained by an increased risk of frost damage (Inouye 2008; Gezon et al. 2016) or may already be at the limits of their phenological plasticity (Scranton and Amarasekare 2017). Lacking these constraints, plants in warmer regions like the SECP may exhibit stronger phenological responses to temperature than those in cooler regions (Menzel et al. 2006). Furthermore, because warm, humid climates like the SECP experience fewer frost days than cool, temperate climates, plants in the former may not have strong phenological chilling requirements, which could otherwise moderate the effects of temperature on phenology (Chmielewski et al. 2011). The SECP, a region of high botanical activity during the past century, provides an ideal system in which to investigate the phenological sensitivities of plants to temperature in a warm, humid climate.
The SECP also offers an opportunity to examine the effects of precipitation on phenology in a climate that shares characteristics of both temperate (e.g., temperature seasonality) and subtropical (e.g., high humidity) regions. Precipitation has little effect on phenology in many temperate (Abu-Asab et al. 2001; Sparks et al. 2006), alpine (Hart et al. 2014), and Mediterranean (Gordo and Sanz 2005) systems, yet precipitation cycles are critical to phenology in tropical regions (Sahagun-Godinez 1996; Zalamea et al. 2011) and grasslands (Lesica and Kittelson 2010; Chen et al. 2014), and precipitation may even outrank temperature in phenological importance in subtropical (Peñuelas et al. 2004) and arid regions (Crimmins et al. 2013). The influence of precipitation in the SECP may be particularly complex (Von Holle et al. 2010), yet this factor has not been thoroughly explored to date (see Park and Schwartz 2015), leaving a gap in our understanding of climatic drivers of phenological events.
The SECP ecoregion, stretching from east Texas to east Massachusetts and south through Florida (Fig. 1), is a biodiversity hotspot and home to over 6000 species of vascular plants, over 25% of which are endemic to the region (Sorrie and Weakley 2006). Nonparallel phenological shifts of plants and interacting taxa such as pollinators or seed dispersers can lead to phenological asynchrony, which may detrimentally alter plant vital rates (Kudo and Ida 2013), cause local extirpation of pollinator species (Burkle et al. 2013), or lead to novel trophic interactions (Liu et al. 2011). Similarly, phenological shifts may alter the availability of insects and fruits as food for migratory birds, which may affect insect or avian populations (Both et al. 2006). This highly biodiverse region already faces critical ecosystem threats (Nordman et al. 2014), and the need to understand the potential influence of phenological shifts on its ecology is clear. By doing so, this study fills a critical geographic knowledge gap and may help predict future challenges for endemic and threatened species in warm, temperate regions.
This study furthermore addresses the currently limited knowledge of the effects of climate change on autumn phenology (Gallinat et al. 2015). Previous research has indicated that spring and autumn phenological events may have contrasting responses to climate change, with fall phenology showing slight to moderate delays while spring-flowering species display phenological advancement with climate warming (Sparks et al. 2000; Gordo and Sanz 2005; Sherry et al. 2007; Jeong et al. 2011; Gill et al. 2015), though some have found opposite (Høye et al. 2013) or no trends for spring- versus fall-flowering species (Bock et al. 2014). Diverging phenological responses to climate warming across seasons could influence associated species such as pollinators by creating gaps in which floral resources are scarce, and shifts in phenological timing could affect inter- and intraspecific competition between both plant and pollinator species. This study capitalizes on the fact that many species in the SECP bloom in the late summer to fall (Wunderlin and Hansen 2011), allowing comparison of shifts in a similar phenological event (i.e., flowering) among species in different seasons.
To examine the effect of climate change on phenology, this study leverages the rich data source of digitized herbarium specimen records. Herbarium specimens are plants that have been collected, pressed, dried, and preserved for sometimes hundreds of years in natural history collections (i.e., herbaria). Each specimen provides a snapshot of the phenological status of a certain species at a certain time and place. Although they were not necessarily collected with the intent to document phenological events, herbarium records have proven to be reliable sources of phenological data that are vital to advancing our understanding of plant phenology on broad spatiotemporal scales (Davis et al. 2015; Willis et al. 2017; Jones and Daehler 2018). With the large amount of digitized specimen data—including specimen images—now available (e.g., via online portals such as iDigBio; idigbio.org), obtaining the statistical power necessary to distinguish phenological trends is more tractable than ever.
Understanding regional and seasonal differences among plant sensitivities to climate change will allow a more nuanced ability to infer mechanisms, predict phenological trajectories, and form hypotheses for future study of phenological shifts in the Anthropocene. The purpose of this study is to determine (1) how peak flowering times of asteraceous plants change with temperature and precipitation in the SECP, and (2) how this relationship differs between spring-flowering and fall-flowering species.
If shifts in flowering time with temperature are conserved among climate types, spring-flowering species in the SECP, although perhaps not fall-flowering species, are expected to flower earlier in warmer temperatures at a rate near 2–3 days/°C (Calinger et al. 2013). If phenological sensitivity to temperature depends more strongly on climate type, such a trend is not expected. Given the impact of precipitation on phenology in subtropical and tropical regions (Peñuelas et al. 2004; Borchert et al. 2005; Zalamea et al. 2011), species in the warm, humid SECP are expected to exhibit a relationship between peak flowering time and precipitation. However, it also is possible that, because most of this region experiences colder winter temperatures than the subtropics and tropics, plant phenology in the SECP may remain more tightly linked to temperature regimes. Regardless of how phenological sensitivities to climate change compare between climate types, differing phenological responses between spring-flowering and fall-flowering species are expected (Sherry et al. 2007; Gill et al. 2015).
Materials and methods
Dataset selection and cleaning
Eleven genera in the sunflower family (Asteraceae) were selected for this study. The Asteraceae is an ideal system for this study because its members are abundant and highly diverse in the SECP; iDigBio, a national aggregator of specimen records, reports over 56,000 Asteraceae specimens collected in Florida alone since 1842 (as of January 2019; idigbio.org), and the Atlas of Florida Plants reports over 430 asteraceous species in Florida (Wunderlin et al. 2018). Many of these species bloom either during the spring-flowering peak (Feb-May) or the fall-flowering peak (Aug-Oct) in the SECP (Wunderlin and Hansen 2011), allowing examination of the effects of climate change on flowering among spring- and fall-flowering species. The 11 genera were selected to maximize (1) number of specimens per genus, (2) representation of taxonomic (i.e., tribal) diversity within the Asteraceae, and (3) diversity of flowering guild (spring vs. fall) and other traits. Within these genera, 81 species were selected to maximize the number of specimens per species and representation of different flowering guilds while avoiding species that flower year-round (S1).
All herbarium specimen records of the 11 selected genera collected in the US Southeastern Coastal Plain states of Alabama, Florida, Georgia, Louisiana, Mississippi, North Carolina, and South Carolina (Fig. 1) were downloaded from the iDigBio online portal (idigbio.org). The northernmost regions of the SECP, including Virginia, Maryland, New Jersey, New York, Connecticut, Rhode Island, and Massachusetts, were excluded to reduce the effect of latitude on statistical results. Although it is not generally considered within the SECP, south Florida was included to maximize sample size. Records from counties not located within the SECP, as informed by state ecoregion maps (Georgia Department of Natural Resources n.d., North Carolina Department of Public Instruction n.d., Riekerk n.d., University of Alabama Department of Geography n.d.), were removed. Taxonomic names of specimen records were standardized using the iPlant Collaborative Taxonomic Name Resolution Service (Boyle et al. 2013), and specimens that were not of one of the 81 selected species were removed. After phenophase determination of all specimens (see below), duplicate specimens, defined as specimens of the same species collected in the same county on the same date, were identified. An average phenophase was assigned to one record in each set of duplicates, and the other duplicate record(s) were removed from the dataset. Although this approach removes more than “true” duplicates—specimens collected on the same date, by the same collector, and from the same population of plants—many specimens (69%) lacked precise latitude and longitude coordinates, making it infeasible to distinguish “true” duplicates from specimens of the same species collected on the same date but in a different part of the county. Furthermore, the number of duplicates that were not “true” was expected to be small, given the sporadic activity of collectors. Specimens that lacked flowers, including those in 100% budding and 100% fruiting phases, were removed. Finally, spatial (i.e., latitudinal or longitudinal) outliers were identified using Cleveland dot plots and removed to prevent disproportional effects of these points on subsequent models (Zuur et al. 2010).
The cleaned dataset consisted of 9938 specimens with USHCN climate data and 9633 specimens with ClimateNA data of 81 species in the 11 genera. Collections spanned a relatively wide temporal range (1891–2014) and spatial range (25.32–36.45°N; –94.02 to –75.80°W).
Determination of specimen phenophases
Many studies of phenology using herbarium specimens have assessed phenophase on a binary scale (e.g., flowers present or absent; Willis et al. 2017), yet this approach may result in coarse estimates of plant flowering times, especially when the flowering duration of the species is long. Similarly, other metrics of phenology such as first flowering date have been shown to be unreliable (Moussus et al. 2010). Peak flowering date was chosen for comparison in this study, as this value is likely to be near the mean flowering time of the population, which is considered the most reliable metric, even for small sample sizes (Miller-Rushing et al. 2008; Moussus et al. 2010). Because many specimens were not collected during peak flowering, numerical phenophases (1–9) were assigned to each specimen based on the percentage of buds, flowers, and fruits present on the specimen using the nearest quartile values (0, 25, 50, 75, 100%) such that the total of all reproductive structures on a specimen equaled 100%. For example, specimens with 100% buds were assigned phenophase 1, specimens with 75% buds and 25% flowers were assigned phenophase 2, and so on. Flowering duration data (described below) was then used to add or subtract days from the collection day of year (DOY; from 1 to 365) of the specimen and thus estimate its day of peak flowering (i.e., phenophase 5; Pearson 2019).
To determine how many days to add or subtract for estimating day of peak flowering, wild populations of one species from each genus were identified within Leon county, Florida, USA during spring (1 species) and late summer to fall 2017 (10 species), and at least 11 individuals of each species were marked prior to or near the beginning of the flowering period. The quartile percentages of buds, flowers, and fruits on the plant were recorded every 3–4 days until the end of the flowering period (100% fruits), and these percentages were converted into phenophases following the same schema applied to herbarium specimens. For each species, a linear mixed-effects model was used to determine the number of days elapsed per phenophase while taking into account different individual starting dates (DOY~phenophase + (1|individual)). The slope of this model was used to adjust the day of flowering for each specimen record to reflect estimated date of peak flowering. For example, the estimated length of each phenophase in the genus Liatris was 2 days, and thus the date of peak flowering for a Liatris specimen in phenophase 8 would be estimated by subtracting 6 days (2 days/phenophase × 3 phenophases) from the collection date.
This method operates under three main assumptions: (1) the relationship between time and phenophase is linear; (2) flowering duration does not vary significantly with location, climate, time, or population; and (3) flowering duration is similar among species within a genus. The data suggest that assumption 1 is reasonable in these species: a significant linear relationship between time and phenophase was discovered for all monitored species (S2), and the distribution of the residuals, visualized in normal QQ plots, did not indicate deviation from normality. Quadratic models were also fit to the data, and AICc values were used to compare goodness of fit of the quadratic models to the linear models. The quadratic models had better fit for two genera, Eupatorium and Marshallia. However, when peak flowering dates of Eupatorium and Marshallia specimens were calculated according to the quadratic relationship, results of subsequent analyses followed similar trends to those reported in Results (S3). With regard to the second assumption, flowering duration is expected to be moderately shorter in warmer regions (Sherry et al. 2011; Bock et al. 2014). Consequently, measures of flowering duration in this study estimated in Florida, the warmest state in the SECP, are expected to be conservative and thus not introduce a large amount of variance. Although data on the effects of climate change on flowering duration is lacking, some comparative (Kang and Jang 2004) and experimental (Gillespie et al. 2016) studies have found no correlation between warmer temperatures and flowering duration. There is some evidence that flowering duration has changed over time in some regions (Bock et al. 2014); however, Bock et al. (2014) only investigated flowering duration on the population level, which provides little evidence that individual rates of progression between phenophases have changed over time. Regarding assumption 3, even between genera, durations of the observed species ranged from 1.6 to 4.4 days per phenophase (S2), indicating that flowering durations are similar within the family, and intrageneric variation in flowering duration is therefore expected to be mild.
Specimens flowering significantly out of season (before DOY 150 for fall-flowering species or after DOY 150 for spring-flowering species) were excluded from per-season analyses, as these individuals were likely responding to cues other than climate such as fire (Conceicao and Orr 2012) or other disturbance.
Climate data
Two approaches to estimating climate parameters were used, and the results of each were compared to assess consistency. The first approach utilized climate data from meteorological stations of the US Climatology Network (USHCN; Menne et al. 2009), which may contain biases (Pielke Sr. et al. 2007) and may not necessarily reflect conditions at the collection location of specimens. The second approach leveraged the ClimateNA application (Wang et al. 2016), which interpolates climate data for specific coordinates but is therefore limited by the accuracy and precision of its underlying models. Using two different sources of climate data in this study reduces the likelihood that conclusions drawn are due to biases or uncertainties in climate data.
In the first approach, bias-averaged monthly average temperature and monthly total precipitation data for all available years were obtained from the USHCN version 2 in February 2017. The R packages sp and rgeos were used to determine the nearest meteorological station to either the collection coordinates provided for each specimen or, if the specimen lacked coordinates (69% of specimens), the centroid of the county in which the specimen was collected. The SECP is topographically homogeneous, and therefore climate is expected to be similar throughout the county in a given month. Specimens that could not be assigned to a county from label data were excluded. Climate data in the collection year from the nearest meteorological station was associated with each specimen record. Specimens with year + station combinations lacking climate data were excluded.
Because latitude may influence flowering time independently of temperature (Molnár et al. 2012; Bjorkman et al. 2017) and temperature was strongly correlated with latitude in the USHCN dataset, temperature deviation rather than absolute temperature was used as a fixed effect in the LME models using the USHCN data. Temperature deviation was calculated as the difference between the actual value of temperature at the latitude of measurement (i.e., climate station) and an expected value calculated using a linear regression of monthly temperature versus latitude. All data were pooled in this linear regression, so the resulting expected values were those for all years and longitudes. In the temperature deviation metric, negative values reflect colder-than-average years and positive values reflect warmer-than-average years. Calculating precipitation deviation was not appropriate because precipitation did not vary as predictably with latitude, and monthly (March or July) total precipitation values were used instead. Although elevation contributes to the timing of phenological events in many systems (Gugger et al. 2015), it was not considered in these analyses because altitudinal variation is minimal across the SECP (Fig. 1).
In the second approach, climate variables were determined using ClimateNA, an application that uses PRISM (Daly et al. 2008) and WorldClim (Hijmans et al. 2005) data to calculate normal monthly climate values (from 1961 to 1990) and historical monthly climate values for given coordinates (i.e., specimen locations). In analyses using these data, the fixed effect of LMEs was calculated as the difference between the climate value in the month of specimen collection (e.g., average temperature in July 1980) and the normal climate value for the specimen’s location (e.g., normal average temperature in July from 1961 to 1990). As before, specimens that could only be georeferenced to the accuracy of county were assigned a location at the center of that county.
Previous studies have indicated that plants are most responsive to climate conditions during the months immediately prior to a phenological event (Menzel et al. 2006; Munson and Sher 2015). Thus, I investigated spring-flowering species’ sensitivities to March climate conditions and fall-flowering species’ sensitivities to July climate conditions. Fall-flowering species’ sensitivities to March climate conditions were also examined for any effects of spring climate on fall phenology.
To determine whether climate has changed with time in this region, each of the climate variables from the USHCN climate dataset (monthly temperature deviation or total precipitation) was regressed with year. Separate models were created for the entire range of dates (1894–2014) and for the range of dates beginning in 1970, which has been suggested as the onset of the most recent, rapid climate warming (Hodgkins et al. 2003). Climate and year data were those associated with specimens in the phenological dataset.
Flowering guild determination
Species were denoted “spring-flowering” if the mean peak flowering date (as determined above) of all specimens of the species was earlier than DOY 150 (May 31). “Fall-flowering” species were those with a mean peak flowering date later than DOY 211 (July 31).
Statistical analyses
Linear mixed-effects (LME) models (lme4 package in R; R Core Team 2016) were used to model the relationship between estimated peak flowering DOY and each climate variable (continuous fixed effect), accounting for differences among species (random effect). It was not necessary to transform DOY to account for flowering across the December 31–January 1 boundary (as in Park and Mazer 2018) because none of the focal species flower across this boundary (e.g., the mean flowering date for the latest-flowering genus was 285 ± 57). As described above (see Climate data ), climate variables using USHCN data were either monthly temperature deviation or total monthly precipitation. Climate variables using ClimateNA data were the difference between year-of-collection monthly temperature/precipitation and normal monthly temperature/precipitation. LMEs allowing both slopes and intercepts to vary between species with temperature (DOY~Temperature + (Temperature|Species)) had no better fit than LMEs allowing only intercepts to vary between species (DOY~Temperature + (1|Species)). LMEs allowing slopes and intercepts to vary with precipitation failed to converge and could not be properly assessed for fit. Thus, for both climate variables (temperature and precipitation), only variable intercept models were used. Fall-flowering and spring-flowering species were modeled separately. In these models, negative values of estimated slope indicate earlier peak flowering date with greater temperature deviations, while positive values of estimated slope indicate later peak flowering dates with warmer temperatures. Reported confidence intervals (CIs) are 95% confidence intervals calculated using the confint function in the stats package of R.
Modeling phenological sensitivity to climate change in this way assumes that species respond similarly across the large spatial range covered by this dataset. Current evidence is mixed regarding the validity of this assumption. Chen and Xu (2012) and Chen et al. (2015) discovered differences in phenological sensitivities to climate within woody and herbaceous species, respectively, at different locations. Conversely, in a case study, Phillimore et al. (2012) discovered no differences in phenological sensitivities to climate variables among locations in two herbaceous species. Toftegaard et al. (2016) found that only 1 of 5 cruciferous species in Sweden showed a slight difference in phenological sensitivity among latitudes, though the potentially important effect of photoperiod (Tooke and Battey 2010) was not accounted for in this study. Plants at the same latitude in the UK and Poland demonstrated dissimilar phenological responses to climate change (Tryjanowski et al. 2006), but contrasting conditions at these two sites (i.e., island vs. mainland climates) may have driven this difference. Climatic conditions within the SECP are, in contrast, similar even across the latitudinal and longitudinal range of this study, and variance in phenological sensitivities to climate across this region is thus expected to be minimal.
Climatic outliers were identified using Cleveland dot plots and removed from the dataset prior to model fitting, as they were likely to represent data quality problems rather than actual climatic conditions. All models were examined for homogeneity of variance and normal distribution of within- and between-group residuals. Statistically significant improvement of model fit was assessed by comparing small-sample-size-corrected Akaike information criterion (AICc) values calculated with the AICc function in the MuMIn package in R.
Results
Climate change in the SECP
Annual, July, and March temperature deviation showed significant, positive relationships with year over the whole time period (1894–2014; Table 1). In the 1970–2014 time period, the rate of change tripled for annual and March temperature deviation and doubled for July temperature deviation such that the average temperature deviation increased 0.18–0.24 °C per year (Table 1; Fig. 2). July and March precipitation, but not annual precipitation, decreased over time over the whole time period. In the 1970–2014 time period, total annual precipitation decreased dramatically at a rate of nearly 2.5 cm per year, and March precipitation decreased 0.5 cm per year. July precipitation did not change significantly over time between 1970 and 2014.
Change in annual temperature deviation over time in the specimen dataset. The dashed red line (crossing entire graph) shows linear regression of annual temperature deviation with year across the entire period (1894–2014), and the yellow line (short line on far right) indicates linear regression during the period of recent, rapid climate warming (1970–2014; Hodgkins et al. 2003)
Phenological sensitivities to climate
Spring- and fall-flowering species showed marked differences in flowering date change with climate variables. When assessed using USHCN climate data, flowering dates of spring-flowering species (890 specimens, 10 species) were earlier with higher March temperature deviation: for every 1 °C increase in March temperature deviation, peak flowering was 1.8 days earlier (95% CI: 1.2, 2.4; Fig. 3). Models using ClimateNA data showed similar results. For every 1 °C above March normal temperature, peak flowering dates of spring-flowering species (876 specimens after removal of specimens lacking adequate climate data) were advanced 2.3 days (95% CI: 1.6, 3.0).
LME models of relationship between day of peak flowering (1–365) and temperature deviation for spring-flowering species (top) and fall-flowering species (bottom) using USHCN climate data. Solid gray lines indicate regressions for each species across the range of temperatures in which it occurred in the dataset, and dashed green or red lines indicate the average for all species across the total range of temperatures. Both spring-flowering and fall-flowering species showed significantly earlier flowering dates with warmer-than-average March temperatures (top and bottom left), but fall-flowering species demonstrated later flowering times with warmer-than-average July temperatures (bottom right). Results of models using ClimateNA data rather than USHCN data are not shown but produced similar results
Because most fall-flowering species in this study are perennial, these plants may respond to climate cues throughout the growing season, rather than only immediately prior to reproduction. Indeed, flowering dates of fall-flowering species were 0.52 days earlier per + 1 °C deviation in March temperature (95% CI 0.27, 0.77) according to USHCN-based models (8700 specimens, 72 species). In contrast, flowering dates of fall-flowering species (8700 specimens, 72 species) were later with increased July temperatures. USHCN-based models showed that peak flowering dates of fall-flowering species were 1.2 days later for every 1 °C increase in July temperature deviation (95% CI 0.69, 1.6; Fig. 3). Again, models using ClimateNA data were similar, indicating a small advance of 0.80 days with every 1 °C warmer-than-normal March temperature (95% CI 0.49, 1.12) and a greater, 2.9 day delay per 1 °C warmer-than-normal July temperature (95% CI 1.40, 2.92; 8473 specimens after removal of specimens lacking adequate climate data).
Spring-flowering species’ flowering dates were a mere 0.24 days later per centimeter increase in March precipitation (95% CI: 0.050, 0.43), and fall-flowering species’ flowering dates were only 0.17 days later per centimeter increase in July precipitation (95% CI: 0.10, 0.24) according to USHCN climate data. ClimateNA data indicated that spring-flowering species flowering times were 2.3 days later per centimeter increase in March precipitation (95% CI 0.15, 4.4), and fall-flowering species’ flowering dates were not significantly affected by July precipitation.
Discussion
Contrary to some previous predictions (e.g., Pau et al. 2011), plant species in the warm temperate climate of the SECP responded to temperature in ways similar to those in cold temperate climates. The 1.8–2.3-day phenological advancement of spring-flowering species per degree March warming shows striking agreement with estimates in, for example, north-central North America (2.4 days/°C; Calinger et al. 2013), northeast North America (3.1 days/°C; Miller-Rushing and Primack 2008), and the UK (1.4–3.4 days/°C; Sparks et al. 2000). Also somewhat unexpectedly, phenological sensitivity to climate change was identified in the Asteraceae, a plant family that has been suggested to track climate variables less strongly than other groups (Davis et al. 2010). These findings suggest that interannual phenological variations—and perhaps many phenological cues—may be reasonably generalizable among climate regions and some taxa, and even warm-adapted species like those in the SECP are not immune to the potential phenological effects of climate warming.
The flora and fauna of the SECP may instead be uniquely threatened given the phenological trends and evidence of climate warming discovered in this study. Flowering times of fall-flowering species were delayed by 1.2–2.9 days per 1 °C July warming, suggesting that these species flower later with warmer-than-average summer temperatures. This response is consistent with the general pattern of fall phenological response to preseason temperature (Walther et al. 2002; Ibañez et al. 2010); however, this study detected this effect within a large number of fall-flowering species rather than in, for example, trends of leaf senescence of deciduous trees (Gill et al. 2015). Von Holle et al. (2010) also found delays in flowering times of 70 plant species in Florida, although these delays were associated with increased minimum temperature variability rather than increased average temperatures.
This study demonstrated that climate warming is evident in the SECP and may have accelerated in the 1970s (Fig. 2), indicating that warming-induced phenological delays and advances inferred here may be currently coming to bear. These phenological shifts could have numerous ecological and evolutionary consequences. Especially when coupled with advances in spring-flowering events, delays in fall flowering could have negative consequences for associated species such as pollinators by creating a longer summer “dead zone” in which floral resources are scarce (Aldridge et al. 2011). Species that depend on the availability of flowers between peak blooms may experience increased competition for floral resources and potentially suffer from decreased fitness and consequent population declines. Similarly, plants that flower before or after most other species in the season may experience changes in abundance and diversity of floral visitors, which could affect fitness and alter selective pressures on phenological traits. Flowering later could also affect fruit dispersal patterns, the phenological overlap of plants with herbivores (Liu et al. 2011), or temporal overlap with climatic conditions. For example, plants that flower later may be more susceptible to flower or fruit damage due to cold conditions in coming winter months, just as flowering too early can predispose spring plants to frost damage (Inouye 2008). Fall phenological events may be just as critical to monitor as events of spring.
An alternative explanation of the results of this study is that the flowering period of fall-flowering species becomes longer with warmer temperatures. In this scenario, mean flowering time (approximated in this study as peak flowering time) would be later with warmer temperatures, as was discovered in this study, yet onset of the fall-flowering period would not be significantly changed. I tested this hypothesis by determining whether variance in flowering date changed significantly with temperature. Increased variance would be expected if the period of flowering was longer because flowering specimens could be collected within a greater span of time around mean flowering. Variance in flowering date did not consistently and significantly change with temperature, and thus the alternative hypothesis was not supported (S4). At least in the SECP, the relationship of fall-flowering species’ flowering date with temperature is likely due to changes in the timing—rather than duration—of the flowering season with temperature. This interpretation has been similarly supported in experimental (Post et al. 2008) and observational studies (Bock et al. 2014).
In addition to delays with warmer-than-average summer temperatures, fall-flowering species experienced a small advance in flowering time in warmer-than-average springs. This could mean that spring temperatures contribute to phenological cuing, perhaps by triggering earlier growth or development. Whatever the mechanism, this contrasting response to different seasonal cues highlights the importance of understanding changes in climate if we are to predict the effects of climate change on phenology in this region. For instance, if this region experiences uniform warming within a year, warm springs may moderate the delaying effect of warm summers for fall-flowering species. Conversely, if plants are exposed to both spring cooling and summer warming, delays in flowering time may be compounded, potentially exacerbating effects on plants, pollinators, and higher trophic levels. Accurately predicting phenological responses of plants and monitoring potential effects will require careful attention to temperature cues across seasons.
Another important consideration from this study is the impact of precipitation on both spring and fall-flowering events. Unlike in many temperate, alpine, and Mediterranean climates (Abu-Asab et al. 2001; Hart et al. 2014; Gordo and Sanz 2005), precipitation was related to timing of flowering for spring-flowering plants in the SECP. In both climate datasets, spring-flowering species bloomed later with increased spring precipitation, albeit by rates as low as 0.24 days later per centimeter. The large difference in estimated rates of phenological change using the USHCN precipitation data and ClimateNA precipitation data (2 days/cm) may indicate that the methods employed in this study (e.g., specimen localities assigned as county centroids, in some cases) are too imprecise to accurately measure the effect of precipitation on phenology here, since precipitation can be highly localized in this region and individual species may respond differently (Von Holle et al. 2010). Nevertheless, later spring flowering with increased precipitation was inferred from both analyses. Fall phenology was less clearly related to precipitation. In the USHCN climate dataset, later flowering dates occurred with warmer July temperatures, while the ClimateNA climate dataset showed no significant relationship of flowering date with July precipitation. This discrepancy may imply more complicated relationships of phenology and precipitation than could be elucidated by this broad-brush approach. Further study on the effect of precipitation on phenology is needed.
For both temperature and precipitation, the season of flowering proved critical to explaining phenological sensitivity to climate change, underlining the importance of considering seasonal phenological events separately rather than assuming a uniform response. Determining plant phenological sensitivities to each of these potential cues is important to understanding potential phenological asynchrony in this region. Although some studies have suggested that the consequences of phenological asynchrony may not be as dire as once believed (Miller-Rushing et al. 2010; Forrest 2015), temporal mismatches with pollinators (Burkle et al. 2013; Kudo and Ida 2013) and increased overlap with herbivores (Liu et al. 2011) may decrease floral fitness (Thomson 2010; Miller-Rushing et al. 2010; Forrest 2015) and negatively impact pollinator populations (Burkle et al. 2013). The high biodiversity of the SECP may render it more vulnerable to species loss due to such change, thus the threat of negative effects of asynchrony must be taken seriously. Critical to the species studied here, Rafferty et al. (2015) predicted that more generalized mutualisms with brief seasonal interactions—characteristics of many asteraceous species in the SECP—are more likely to become unsynchronized with other ecologically important species and may be in greater peril of possible detrimental effects. Timing with abiotic factors such as frost, storms (e.g., hurricanes, which are frequent in the SECP), and wind may also play a key role in determining population success. Rapid evolution of phenological traits under such potentially strong selective processes is possible (Franks et al. 2007), but the capacity of these taxa and their interacting species to adapt quickly enough to avoid substantial fitness losses is uncertain.
Assessing phenological sensitivities of plant species to climate change using herbarium specimen data has limitations due to, for example, the coarse spatial granularity of some specimen locality data and the lack of repeated measures of phenology at identical sites. Experimental and observational studies are needed to further examine the effect of traits and different climatic cues on plant phenological change. Nevertheless, this and similar studies provide critical data from which hypotheses can be formed and spatiotemporal trends can be extracted on a much larger scale than is feasible for many experimental and observational studies (Willis et al. 2017). Efforts to obtain a similar scale of data via citizen science are underway (e.g., National Phenology Network; usanpn.org; Schwartz et al. 2012; Project Budburst, budburst.org; European Phenology Campaign, globe.gov/web/european-phenology-campaign), and combining observational and specimen-based records may prove a powerful way forward for understanding phenological change (Spellman and Mulder 2016). Still, these new datasets lack the historical record of phenological events that herbarium specimens possess. With increasing availability of specimen data through digitization, development of protocols and standards for better integration of specimen-based phenological data (e.g., Yost et al. 2018), and development of statistical techniques to account for data limitations (Pearse et al. 2017), specimen data present ever-increasing opportunities to examine phenological trends and direct mitigation of adverse biotic change.
References
Abu-Asab MS, Peterson PM, Shetler SG, Orli SS (2001) Earlier plant flowering in spring as a response to global warming in the Washington, DC, area. Biodivers Conserv 10:597–612
Aldridge G, Inouye DW, Forrest JRK, Barr WA, Miller-Rushing AJ (2011) Emergence of a mid-season period of low floral resources in a montane meadow ecosystem associated with climate change. J Ecol 99:905–913
Bjorkman AD, Vellend M, Frei ER, Henry GHR (2017) Climate adaptation is not enough: warming does not facilitate success of southern tundra plant populations in the high Arctic. Glob Change Biol 23:1540–1551
Bock A, Sparks TH, Estrella N, Jee N, Casebow A, Schunk C, Leuchner M, Menzel A (2014) Changes in first flowering dates and flowering duration of 232 plant species on the island of Guernsey. Glob Change Biol 20:3508–3519
Borchert R, Robertson K, Schwartz MD, Williams-Linera G (2005) Phenology of temperate trees in tropical climates. Int J Biometeorol 50:57–65
Both C, Bouwhuis S, Lessells CM, Visser ME (2006) Climate change and population declines in a long-distance migratory bird. Nature 441:81–83
Boyle B, Hopkins N, Lu Z, Raygoza Garay JA, Mozzherin D, Rees T, Matasci N, Narro ML, Piel WH, Mckay SJ, Lowry S, Freeland C, Peet RK, Enquist BJ (2013) The taxonomic name resolution service: an online tool for automated standardization of plant names. BMC Bioinform 14:16
Burkle LA, Marlin JC, Knight TM (2013) Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339:1611–1615
Calinger KM, Queenborough S, Curtis PS (2013) Herbarium specimens reveal the footprint of climate change on flowering trends across north-central North America. Ecol Lett 16:1037–1044
Chen X, Li J, Xu L, Liu L, Ding D (2014) Modeling Greenup date of dominant grass species inthe inner Mongolian grassland using air temperature and precipitation data. Int J Biometeorol 58:463–471
Chen XQ, Tian YH, Xu L (2015) Temperature and geographic attribution of change in the Taraxacum mongolicum growing season from 1990 to 2009 in eastern China's temperate zone. Int J Biometeorol 59(10):1437–1452
Chen X, Wang L, Inouye D (2017) Delayed response of spring phenology to global warming in subtropics and tropics. Agr Forest Meteorolog 234-235:222–235
Chen XQ, Xu L (2012) Phenological responses of Ulmus pumila (Siberian elm) to climate change in the temperate zone of China. Int J Biometeorol 56(4):695–706
Chmielewski F, Blümel K, Henniges Y, Blanke M, Weber RWS, Zoth M (2011) Phenological models for the beginning of apple blossom in Germany. Meterolog Z 20(5):487–496
Cleland EE, Chiariello NR, Loarie SR, Mooney HA, Field CB (2006) Diverse responses of phenology to global changes in a grassland ecosystem. P Natl Acad Sci USA 103(37):13740–13744
Conceicao AA, Orr BJ (2012) Post-fire flowering and fruiting in Vellozia sincorana, a caulescent rosette plant endemic to Northeast Brazil. Acta Bot Bras 26(1):94–100
Crimmins TM, Crimmins MA, Bertelsen CD (2013) Spring and summer patterns in flowering onset, duration, and constancy across a water-limited gradient. Am J Bot 100(6):1137–1147
Daly C, Halbleib M, Smith JI, Gibson WP, Doggett MK, Taylor GH, Curtis J (2008) Physiographically sensitive mapping of temperature and precipitation across the conterminous United States. Int J Climatol 28:2031–2064
Davis CC, Willis CG, Connolly B, Kelly C, Ellison AM (2015) Herbarium records are reliable sources of phenological change driven by climate and provide novel insights into species’ phenological cueing mechanisms. Am J Bot 102(10):1599–1609
Davis CC, Willis CG, Primack RB, Miller-Rushing AJ (2010) The importance of phylogeny to the study of phenological response to global climate change. Philos T R Soc B 365:3201–3213
Ellwood ER, Playfair SR, Polgar CA, Primack RB (2014) Cranberry flowering times and climate change in southern Massachusetts. Int J Biometeorol 58:1693–1697
Fitter AH, Fitter RSR (2002) Rapid changes in flowering time in British plants. Science 296:1689–1691
Forrest JRK (2015) Plant-pollinator interactions and phenological change: what can we learn about climate impacts from experiments and observations? Oikos 124:4–13
Franks SJ, Sim S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Philos T R Soc B 104:1278–1282
Gaira KS, Rawal RS, Rawat B, Bhatt ID (2014) Impact of climate change on the flowering of Rhododendron arboretum in central Himalaya, India. Curr Sci 106(12):1735–1738
Gallinat AS, Primack RB, Wagner DL (2015) Autumn, the neglected season in climate change research. Trends Ecol Evol 30(3):169–176
Georgia Department of Natural Resources. (n.d.) Geographic regions of Georgia, with counties. Retrieved from http://georgiainfo.galileo.usg.edu/images/uploads/gallery/GeographicRegions.jpg
Gezon ZJ, Inouye DW, Irwin RE (2016) Phenological change in a spring ephemeral: implications for pollination and plant reproduction. Glob Change Biol 22:1779–1793
Gill AJ, Gallinat AS, Sanders-DeMott R, Rigden AJ, Short Gianotti DJ, Mantooth JA, Templer PH (2015) Changes in autumn senescence in northern hemisphere deciduous trees: a meta-analysis of autumn phenology studies. Ann Bot 116:875–888
Gillespie MAK, Baggesen N, Cooper EJ (2016) High Arctic flowering phenology and plant-pollinator interactions in response to delayed snow melt and simulated warming. Environ Res Lett 11:115006
Gordo O, Sanz JJ (2005) Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia 146(3):484–495
Gordo O, Sanz JJ (2010) Impact of climate change on plant phenology in Mediterranean ecosystems. Glob Change Biol 16:1082–1106
Gugger S, Kesselring H, Stöcklin J, Hamann E (2015) Lower plasticity exhibited by high- versus mid-elevation species in their phenological responses to manipulated temperature and drought. Ann Bot 116(6):953–962
Hart R, Salick J, Ranjitkar S, Xu J (2014) Herbarium specimens show contrasting phenological responses to Himalayan climate. P Natl Acad Sci USA 111(29):10615–10619
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hodgkins GA, Dudley RW, Huntington TG (2003) Changes in the timing of high river flows in New England over the 20th century. J Hydrol 278:244–252
Høye TT, Post E, Schmidt NM, Trøjelsgaard K, Forchhammer MC (2013) Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nat Clim Chang 3:759–763
Ibañez I, Primack RB, Miller-Rushing AJ, Ellwood E, Higuchi H, Don Lee S, Kobori H, Silander JA (2010) Forecasting phenology under global warming. Philos T R Soc B 365:3247–3260
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecol 89(2):353–362
Jeong S, Ho C, Gim H, Brown ME (2011) Phenology shifts at start vs. end of growing season in temperate vegetation over the northern hemisphere for the period 1982-2008. Glob Change Biol 17:2385–2399
Jones CA, Daehler CC (2018) Herbarium specimens can reveal impacts of climate change on plant phenology; a review of methods and applications. PEERJ 6:e4576
Kang H, Jang J (2004) Flowering patterns among angiosperm species in Korea: diversity and constraints. J Plant Biol 47(4):348–355
Kharouba HM, Vellend M (2015) Flowering time of butterfly nectar food plants is more sensitive to temperature than the timing of butterfly adult flight. J Anim Ecol 84:1311–1321
Kudo G, Ida TY (2013) Early onset of spring increases the phenological mismatch between plants and pollinators. Ecol 94(10):2311–2320
Lavoie C, Lachance D (2006) A new herbarium-based method for reconstructing the phenology of plant species across large areas. Am J Bot 943(4):512–516
Lesica P, Kittelson PM (2010) Precipitation and temperature are associated with advanced flowering phenology in a semi-arid grassland. J Arid Environ 74:1013–1017
Liu Y, Reich PB, Li G, Sun S (2011) Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecol 92(6):1201–1207
Menne MJ, Williams CN, Vose RS (2009) The U.S. historical climatology network monthly temperature data, version 2. B Am Meteorol Soc 90(7):993–1007
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Braslavska O, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl Å, Defila C, Donnelly A, Filella Y, Jatczak K, Mage F, Mestre A, Nordli Ø, Peñuelas J, Pirinen P, Remišova V, Scheifinger H, Striz M, Susnik A, Van Vliet AJH, Wielgolaski F, Zach S, Zust A (2006) European phenological response to climate change matches the warming pattern. Glob Change Biol 12:1969–1976
Miller-Rushing AJ, Primack RB, Primack D, Mukunda S (2006) Photographs and herbarium specimens as tools to document phenological changes in response to global warming. Am J Bot 93(11):1667–1674
Miller-Rushing AJ, Primack RB (2008) Global warming and flowering times in Thoreau’s Concord: a community perspective. Ecol 89(2):332–341
Miller-Rushing AJ, Inouye DW, Primack RB (2008) How well do first flowering dates measure plant responses to climate change? The effects of population size and sampling frequency. J Ecol 96:1289–1296
Miller-Rushing AJ, Høye TT, Inouye DW, Post E (2010) Effects of phenological mismatches on demography. Philos T R Soc B 365:3177–3186
Molnár VA, Tökölyi J, Végvári Z, Sramkó J, Barta Z (2012) Pollination mode predicts phenological response to climate change in terrestrial orchids: a case study from Central Europe. J Ecol 100:1141–1152
Moussus J, Julliard R, Jiguet F (2010) Featuring 10 phenological estimators using simulated data. Methods Ecol Evol 1:140–150
Munson SM, Long AL (2016) Climate drives shifts in grass reproductive phenology across the western USA. New Phytol:1–11
Munson SM, Sher AA (2015) Long-term shifts in the phenology of rare and endemic Rocky Mountain plants. Am J Bot 102(8):1268–1276
Neil KL, Landrum L, Wu J (2010) Effects of urbanization on flowering phenology in the metropolitan phoenix region of USA: findings from herbarium records. J Arid Environ 74:440–444
Nordman CW, Pyne M, Smyth RL, White R (2014) Threats to ecological systems in the South Atlantic landscape conservation cooperative area. Nature Serve. Retrieved from http://data.southatlanticlcc.org/Threats_to_Ecological_Systems_in_SALCC.pdf
North Carolina Department of Public Instruction (n.d.) [Map of North Carolina ecoregions and counties]. North Carolina Regions. Retrieved from http://www.ncpublicschools.org/curriculum/socialstudies/elementary/studentsampler/20geography#maps
Pan C, Feng Q, Zhao H, Liu L, Li Y, Li Y, Zhang T, Yu X (2017) Earlier flowering did not alter pollen limitation in an early flowering shrub under short-term experimental warming. Sci Rep 7(2795):1–5
Panchen ZA, Primack RB, Anisko T, Lyons RE (2012) Herbarium specimens, photographs, and field observations show Philadelphia area plants are responding to climate change. Am J Bot 99(4):751–756
Park IW, Mazer SJ (2018) Overlooked climate parameters best predict flowering onset: assessing phenological models using the elastic net. Glob Change Biol 24:5972–5984
Park IW, Schwartz MD (2015) Long-term herbarium records reveal temperature-dependent changes in flowering phenology in the southeastern USA. Int J Biometeorol 59:347–355
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Ann Rev Ecol Evol S 37:637–669
Pau S, Wolkovich EM, Cook BI, Davies TJ, Kraft NJB, Bolmgren K, Betancourt JL, Cleland EE (2011) Predicting phenology by integrating ecology, evolution, and climate science. Glob Change Biol 17:3633–3643
Pearse WD, Davis CC, Inouye DW, Primack RB, Davies TJ (2017) A statistical estimator for determining the limits of contemporary and historic phenology. Nat Ecol Evol 1:1876–1882
Pearson KD (2019) A new method and insights for estimating phenological events from herbarium specimens. Appl Plant Sci (in press)
Peñuelas J, Filella I, Zhang X, Llorens L, Ogaya R, Lloret F, Comas P, Estiarte M, Terradas J (2004) Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytol 161:837–846
Phillimore AB, Stålhandske S, Smithers RJ, Bernard R (2012) Dissecting the contributions of plasticity and local adaptation to the phenology of a butterfly and its host plants. Am Nat 180(5):655–670
Pielke R Sr, Nielsen-Gammon J, Davey C, Angel J, Bliss O, Doesken N, Cai M, Fall S, Niyogi D, Gallo K, Hale R, Hubbard KG, Lin X, Li H, Raman S (2007) Documentation of uncertainties and biases associated with surface temperature measurement sites for climate change assessment. Bull Amer Meteor Soc 88:913–928
Posledovich D, Toftegaard T, Wiklund C, Ehrlén J, Gotthard K (2017) Phenological synchrony between a butterfly and its host plants: experimental test of effects of spring temperature. J Anim Ecol 87:150–161
Post ES, Pedersen C, Wilmers CC, Forchhammer MC (2008) Phenological sequences reveal aggregate life history response to climatic warming. Ecol 89(2):363–370
Price M, Waser NM (1998) Effects of experimental warming on plant reproductive phenology on a subalpine meadow. Ecol 79(4):1261–1271
Primack D, Imbres C, Primack RB, Miller-Rushing AJ, Del Tredici P (2004) Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. Am J Bot 91(8):1260–1264
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ Version 3.3.2.
Rafferty NE, CaraDonna PJ, Bronstein JL (2015) Phenological shifts and the fate of mutualisms. Oikos 124:14–21
Richardson AD, Black TA, Ciais P, Delbart N, Friedl MA, Gabron N, Hollinger DY, Kutsch WL, Longodz B, Luyssaert S, Migliavacca M, Montagnani L, Munger JW, Moors E, Piao S, Rebmann C, Reichstein M, Saigusa N, Tomelleri E, Vargas R, Varlagin A (2010) Influence of spring and autumn phenological transitions on forest ecosystem productivity. Phil T R Soc B 365:3227–3246
Riekerk, G (n.d.) [map of South Carolina coastal plain] South Carolina coastal plain. SCDNR marine resources research institute. Retrieved from https://webapp1.dlib.indiana.edu/virtual_disk_library/index.cgi/4928836/FID1596/html/envicond/geomorph/gmmorph.htm
Rumpff L, Coates F, Morgan JW (2010) Biological indicators of climate change: evidence from long-term flowering records of plants along the Victorian coast, Australia. Aust J Bot 58:428–439
Sahagun-Godinez E (1996) Trends in the phenology of flowering in the Orchidaceae of western Mexico. Biotropica 28(1):130–136
Schwartz MD, Betancourt JL, Weltzin JF (2012) From Caprio’s lilacs to the USA National Phenology Network. Front Ecol Environ 10:324–327
Scranton K, Amarasekare P (2017) Predicting phenological shifts in a changing climate. P Natl Acad Sci USA 114(50):13212–13217
Sherry RA, Zhou X, Gu S, Arnone JA III, Johnson DW, Schimel DS, Verburg PSJ, Wallace LL, Luo Y (2011) Changes in duration of reproductive phases and lagged phenological response to experimental climate warming. Plant Ecol Divers 4(1):23–35
Sherry RA, Zhou X, Gu S, Arnone JA III, Schimel DS, Verburg PS, Wallace LL, Luo Y (2007) Divergence of reproductive phenology under climate warming. P Natl Acad Sci USA 104(1):198–202
Sorrie BA, Weakley AS (2006) Conservation of the endangered Pinus palustris ecosystem based on coastal plain centres of plant endemism. Appl Veg Sci 9:59–66
Sparks TH, Jeffree EP, Jeffree CE (2000) An examination of the relationship between flowering times and temperature at the national scale using long-term phenological records from the UK. Int J Biometeorol 44:82–87
Sparks TH, Huber K, Croxton PJ (2006) Plant development scores from fixed-date photographs: the influence of weather variables and recorder experience. Int J Biometeorol 50:275–279
Spellman KV, Mulder CPH (2016) Validating herbarium-based phenology models using citizen-science data. Bioscience 66:807–906
Tansey CJ, Hadfield JD, Phillimore AB (2017) Estimating the ability of plants to plastically track temperature-mediated shifts in the spring phenological optimum. Glob Change Biol 23:3321–3334
Thomson JD (2010) Flowering phenology, fruiting success and progressive deterioration of pollination in an early-flowering geophyte. Philos T R Soc B 365:3187–3199
Toftegaard T, Posledovich D, Navarro-Cano JA, Wiklund C, Gotthard K, Ehrlén J (2016) Variation in plant thermal reaction norms along a latitudinal gradient—more than adaptation to season length. Oikos 125:622–628
Tooke F, Battey NH (2010) Temperate flowering phenology. J Exp Bot 61(11):2853–2862
Tryjanowski P, Panek M, Sparks T (2006) Phenological response of plants to temperature varies at the same latitude: case study of dog violet and horse chestnut in England and Poland. Clim Res 32:89–93
University of Alabama Department of Geography (n.d.) [Graph illustration of physiography and counties of Alabama] General Physiography of Alabama. Retrieved from http://alabamamaps.ua.edu/contemporarymaps/alabama/physical/basemap6.pdf
Von Holle B, Wei Y, Nickerson D (2010) Climatic variability leads to later seasonal flowering of Floridian plants. PLoS One 5(7):e11500
Walther G, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Wang T, Hamann A, Spittlehouse D, Carroll C (2016) Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLoS One 11:e0156720
Willis CG, Ellwood ER, Primack RB, Davis CC, Pearson KD, Gallinat AS, Yost JM, Nelson G, Mazer SJ, Rossington NL, Sparks TH, Soltis PS (2017) Old plants, new tricks: phenological research using herbarium specimens. Trends Ecol Evol 32(7):531–546
Wunderlin RP, Hansen BF (2011) Guide to the vascular plants of Florida, Third edn. University Press of Florida, Gainesville, FL
Wunderlin RP, Hansen BF, Franck AR, Essig FB (2018) Atlas of Florida plants (http://florida.plantatlas.usf.edu/).[S. M. Landry and K. N. Campbell (application development), USF water institute.] Institute for Systematic Botany, University of South Florida, Tampa
Yost JM, Sweeney PW, Gilbert E, Gilson G, Guralnick R, Gallinat AS, Ellwood ER, Rossington N, Willis CG, Blum SD, Walls RL, Haston EM, Denslow MW, Zohner CM, Morris AB, Stucky BJ, Carter JR, Baxter DG, Bolmgren K, Denny EG, Dean E, Pearson KD, Davis CC, Mishler BD, Soltis PS, Mazer SJ (2018) Digitization protocol for scoring reproductive phenology from herbarium specimens of seed plants. App Plant Sci 6(2):e1022
Zalamea P, Munoz F, Stevenson PR, Paine CET, Sarmiento C, Sabatier D, Heuret P (2011) Continental-scale patterns in Cecropia reproductive phenology: evidence from herbarium specimens. P R Soc B 278(1717):2437–2445
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgments
The author thanks Austin Mast, Gil Nelson, Greg Riccardi, and Libby Ellwood for providing support, advice, and guidance during this project, as well as two anonymous reviewers for critical comments on an earlier manuscript. Special thanks to Scott Burgess for help with statistics, modeling, and R code and to Natali Ramirez-Bullon, Blair Pearson, and Molly Wiebush for field work.
Funding
This research was supported by the Botanical Society of America, American Society of Plant Taxonomists, Florida State University Robert K. Godfrey Award, and through iDigBio, which is funded by a grant from the National Science Foundation’s Advancing Digitization of Biodiversity Collections Program (award number 1547229). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Appropriate permits were acquired to conduct phenological monitoring in Apalachicola National Forest.
Conflict of interest
The author declares that they have no conflict of interest.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pearson, K.D. Spring- and fall-flowering species show diverging phenological responses to climate in the Southeast USA. Int J Biometeorol 63, 481–492 (2019). https://doi.org/10.1007/s00484-019-01679-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-019-01679-0