Abstract
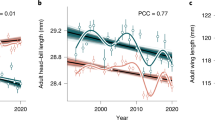
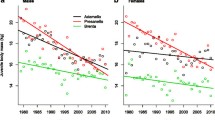
Climate change appears to affect body size of animals whose optimal size in part depends on temperature. However, attribution of observed body size changes to climate change requires an understanding of the selective pressures acting on body size under different temperatures. We examined the link between temperature and body mass in a population of mountain wagtails (Motacilla clara) in KwaZulu-Natal, South Africa, between 1976 and 1999, where temperature increased by 0.18 \(^\circ \)C. The wagtails became lighter by 0.035 g per year. Partitioning this trend, we found that only a small part of the effect (0.009 g/year) was due to individuals losing weight and a large part (0.027 g/year) was due to lighter individuals replacing heavier ones. Only the latter component was statistically significant. Apparently, the wagtails were reacting to selection for reduced weight. Examining survival, we found that selection was temperature-mediated, i.e., lighter individuals survived better under high temperatures, whereas heavier individuals survived better under low temperatures. Our results thus support the hypothesis that temperature drove the decline in body mass in this wagtail population and provides one of the first demonstrations of the selective forces underlying such trends.





Similar content being viewed by others
References
Ashton KG (2002) Patterns of within-species body size variation of birds: strong evidence for Bergmann’s rule. Glob Ecol Biogeogr 11:505–523
Ashton KG, Tracy MC, Queiroz AD (2000) Is Bergmann’s rule valid for mammals? Am Nat 156:390–415
Bergmann C (1847) Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien 3:595–708
Blackburn TM, Gaston KJ, Loder N (1999) Geographic gradients in body size: a clarification of Bergmann’s rule. Divers Distrib 5:165–174
Blunden J, Arndt DS (2017) State of the climate in 2016. Bull Am Meteorol Soc 98:Si-S277
Both C, te Marvelde L (2007) Climate change and timing of avian breeding and migration throughout Europe. Clim Res 35:93–105
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Calder WA (1983) Ecological scaling: mammals and birds. Annu Rev Ecol Syst 14:213–230
Duckworth GD, Altwegg R (2014) Environmental drivers of an urban Hadeda Ibis population. Ardea 102:21–29
Durbin J, Koopman SJ (2012) Time series analysis by state space methods, 2nd edn. Oxford University Press, Oxford
Duriez O, Ens BJ, Choquet R, Pradel R, Klaassen M (2012) Comparing the seasonal survival of resident and migratory oystercatchers: carry-over effects of habitat quality and weather conditions. Oikos 121:862–873
Engelbrecht FA, Mcgregor JL, Engelbrecht CJ (2009) Dynamics of the conformal-cubic atmospheric model projected climate-change signal over southern Africa. Int J Climatol 1033:1013–1033
Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R (2011) Declining body size: a third universal response to warming? Trends Ecol Evol 26:285–291
Gardner JL, Rowley E, de Rebeira P, de Rebeira A, Brouwer L (2017) Effects of extreme weather on two sympatric Australian passerine bird species. Philos Trans R Soc B Biol Sci 372:20160148
Goodman RE, Lebuhn G, Seavy NE, Gardali T, Bluso-Demers JD (2012) Avian body size changes and climate change: warming or increasing variability? Glob Change Biol 18:63–73
Husby A, Hille SM, Visser ME (2011) Testing mechanisms of Bergmann’s rule: phenotypic decline but no genetic change in body size in three passerine bird populations. Am Nat 178:202–13
Kleiber M (1932) Body size and metabolism. Hilgardia 6:315–353
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Lindstedt SL, Boyce MS (1985) Seasonality, fasting endurance, and body size in mammals. Am Nat 125:873–878
Martineau L, Larochelle J (1988) The cooling power of pigeon legs. J Exp Biol 136:193–208
McKechnie AE, Wolf BO (2010) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 6:253–6
McKechnie AE, Whitfield MC, Smit B, Gerson AR, Smith EK, Talbot WA, McWhorter TJ, Wolf BO (2016) Avian thermoregulation in the heat: efficient evaporative cooling allows for extreme heat tolerance in four southern hemisphere columbids. J Exp Biol 219:2145–2155
McNab BK (1971) On the ecological significance of Bergmann’s rule. Ecology 52:845–854
McNab BK (2010) Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia 164:13–23
Ozgul A, Tuljapurkar S, Benton TG, Pemberton JM, Clutton-Brock TH, Coulson T (2009) The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325:464–467
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Petris G (2010) An R package for dynamic linear models. J Stat Softw 36:1–16
Petris G, Petrone S (2011) State space models in R. J Stat Softw 41:1–25
Piper SE (1990) Longtailed wagtail Motacilla clara. In: Ginn PJ, McIlleron G, Milstein P le S (eds) The complete book of southern African birds. Struik, Cape Town, pp 376–377
Piper SE (2002) Survival of adult, territorial longtailed wagtails Motacilla clara: the effects of environmental factors and individual covariates. J Appl Stat 29:107–124
R Core Team (2017) R: a language and environment for statistical computing
Rensch B (1938) Some problems of geographical variation and species-formation. Proc Linn Soc Lond 150:275–285
Salewski V, Siebenrock KH, Hochachka WM, Woog F, Fiedler W (2014) Morphological change to birds over 120 years is not explained by thermal adaptation to climate change. PLoS One 9:1–14
Speakman JR, Król E (2010) Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J Anim Ecol 79:726–46
Tattersall GJ, Arnaout B, Symonds MR (2017) The evolution of the avian bill as a thermoregulatory organ. Biol Rev 92:1630–1656
Teplitsky C, Millien V (2014) Climate warming and Bergmann’s rule through time: is there any evidence? Evol Appl 7:156–168
Teplitsky C, Mills JA, Alho JS, Yarrall JW, Merila J, Merilä J (2008) Bergmann’s rule and climate change revisited: disentangling environmental and genetic responses in a wild bird population. Proc Natl Acad Sci 105:13492–13496
Thomas CD, Lennon JJ (1999) Birds extend their ranges northwards. Nature 399:213
Van Buskirk J, Mulvihill RS, Leberman RC (2010) Declining body sizes in North American birds associated with climate change. Oikos 119:1047–1055
van de Pol M, Wright J (2009) A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav 77:753–758
van Gils JA, Lisovski S, Lok T, Meissner W, Ożarowska A, de Fouw J, Rakhimberdiev E, Soloviev MY, Piersma T, Klaassen M (2016) Body shrinkage due to arctic warming reduces red knot fitness in tropical wintering range. Science 352:819–821
Visser ME, Both C, Lambrechts MM (2004) Global climate change leads to mistimed avian reproduction. Birds Clim Change Adv Ecol Res 35:89–110
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Watt C, Mitchell S, Salewski V (2010) Bergmann’s rule; a concept cluster? Oikos 119:89–100
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:S120–139
Whitfield MC, Smit B, McKechnie AE, Wolf BO (2015) Avian thermoregulation in the heat: scaling of heat tolerance and evaporative cooling capacity in three southern African arid-zone passerines. J Exp Biol 218:1705–1714
Wood SN (2006) Generalized additive models: an introduction with R. Chapman and Hall, Boca Raton
Woodward G, Ebenman B, Emmerson M, Montoya JM, Olesen JM, Valido A, Warren PH (2005) Body size in ecological networks. Trends Ecol Evol 20:402–409
Yom-Tov Y (2001) Global warming and body mass decline in Israeli passerine birds. Proc R Soc Lond B 268:947–52
Acknowledgements
This paper is dedicated to the memory of Steven E. Piper who initiated this project and collected the data. This paper is largely based on a presentation Steven gave at the Pan-African Ornithological Conference in 2008. We thank the National Research Foundation of South Africa (Grants 85802 and 114696) and the Alliance for Collaboration on Climate and Earth System Sciences for funding. The NRF accepts no liability for opinions, findings, and conclusions or recommendations expressed in this publication.
Author information
Authors and Affiliations
Contributions
ZB, HB, and RA conceptualized this study based on ideas of the late Steven Piper. ZB and HB collected data. JP, RA, and BE analysed the data. JP and RA wrote the manuscript. All authors contributed to revisions.
Corresponding author
Ethics declarations
Ethical standards
All applicable institutional and national guidelines for the care and use of animals were followed.
Additional information
Communicated by Christopher Whelan.
Electronic supplementary material
The supplementary information contains the R code used to fit state-space models to the temperature and rainfall data, and the analysis of trends in the mass of mountain wagtails.
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prokosch, J., Bernitz, Z., Bernitz, H. et al. Are animals shrinking due to climate change? Temperature-mediated selection on body mass in mountain wagtails. Oecologia 189, 841–849 (2019). https://doi.org/10.1007/s00442-019-04368-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04368-2