Abstract
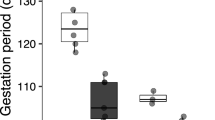
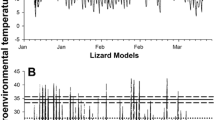
Lizards may experience population declines and extinctions on a similar scale to that experienced by amphibians, and climate warming is one hypothesis proposed to explain these declines and extinctions. Within lizards, viviparous species are hypothesized to be more vulnerable to climate warming, because they have evolved reduced body temperature and heat tolerance, but this idea remains untested. To test this hypothesis, we conducted three temperatures (20, 24, and 28 °C) × two species [Phrynocephalus przewalskii (oviparous) and P. putjatia (viviparous)] factorial design experiment that simulated warming on oviparous versus viviparous lizards. Our manipulation of ambient temperature affected activity and thermal preference in both species, birth date in P. putjatia, and egg mass in P. przewalskii; other examined traits (fecundity, reproductive output, and size, morphology, and sprint speed of offspring) were not affected. Neither in P. putjatia nor in P. przewalskii behavioral responses to rising temperatures differ between the sexes. The viviparous species thermoregulated more actively than did the oviparous species, but the two species did not differ in thermal preference. Warming reduced the activity time allotted for thermoregulation in both species, but the effect was more dramatic in the viviparous species. Our data support one of the central predictions that lead to the hypothesis that viviparous lizards are more vulnerable to climate warming; however, this is not because viviparous lizards have evolved reduced body temperature and heat tolerance, but, because warming constrains activity more dramatically in viviparous species.

Similar content being viewed by others
References
Andrews RM, Mathies T (2000) Natural history of reptilian development: constraints on the evolution of viviparity. Bioscience 50:227–238
Angilletta MJ Jr, Hill T, Robson MA (2002a) Is physiological performance optimized by thermoregulatory behavior?: a case study of the eastern fence lizard, Sceloporus undulatus. J Therm Biol 27:199–204
Angilletta MJ Jr, Niewiarowski PH, Navas CA (2002b) The evolution of thermal physiology in ectotherms. J Therm Biol 27:249–268
Bakken GS (1992) Measurement and application of operative and standard operative temperatures in ecology. Am Zool 32:194–216
Barabanov AV, Ananjeva NB (2007) Catalogue of the available scientific species group names for lizards of the genus Phrynocephalus Kaup, 1825 (Reptilia, Sauria, Agamidae). Zootaxa 1399:1–57
Bauwens D, Thoen C (1981) Escape tactics and vulnerability to predation associated with reproduction in the lizard Lacerta vivipara. J Anim Ecol 50:733–743
Bennett AF, John-Alder HB (1986) Thermal relations of some Australian skinks (Sauria: Scincidae). Copeia 1986:57–64
Beuchat CA (1988) Temperature effects during gestation in a viviparous lizard. J Therm Biol 13:135–142
Bickford D, Howard SD, Ng DJJ, Jennifer A (2010) Impacts of climate change on the amphibians and reptiles of Southeast Asia. Biodivers Conserv 19:1043–1062
Blackburn DG (2000) Reptilian viviparity: past research, future directions, and appropriate models. Comp Biochem Physiol A 127:391–409
Braña F (1993) Shifts in body temperature and escape behaviour of female Podarcis muralis during pregnancy. Oikos 66:216–222
Cadby CD, While GM, Hobday AJ, Uller T, Erik Wapstra E (2010) Multi-scale approach to understanding climate effects on offspring size at birth and date of birth in a reptile. Integr Zool 5:164–175
Carey C (2009) The impacts of climate change on the annual cycles of birds. Phil Trans R Soc B 364:3321–3330
Carey C, Alexander MA (2003) Climate change and amphibian declines: is there a link? Divers Distrib 9:111–121
Chamaillé-Jammes S, Massot M, Aragón P, Clobert J (2006) Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara. Glob Change Biol 12:392–402
Clusella-Trullas S, Chown SL (2011) Comment on ‘‘Erosion of lizard diversity by climate change and altered thermal niches’’. Science 332:537
Clusella-Trullas S, Chown SL (2014) Lizard thermal trait variation at multiple scales: a review. J Comp Physiol B 184:5–21
Congdon JD, Dunham AE, Tinkle DW (1982) Energy budgets and life histories of reptiles. In: Gans C, Pough FH (eds) Biology of reptilia, vol 13. Academic, London, pp 233–271
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672
Dubois Y, Blouin-Demers G, Thomas D (2008) Temperature selection in wood turtles (Glyptemys insculpta) and its implications for energetics. Ecoscience 15:398–406
Dufaure JP, Hubert J (1961) Table de développement du lézard vivipare: Lacerta (Zootoca) vivipara Jacquin. Arch Anat Micr Morph Exp 50:309–328
Feldman A, Bauer AM, Castro-Herrera F, Chirio L, Das I, Doan TM, Maza E, Meirte D, de Campos Nogueira C, Nagy ZT, Torres-Carvajal O, Uetz P, Meiri S (2015) The geography of snake reproductive mode: a global analysis of the evolution of snake viviparity. Glob Ecol Biogeogr 24:1433–1442
Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville TD, Metts BS, Greene JL, Mills T, Leiden Y, Poppy S, Winne CT (2000) The global decline of reptiles, déjà vu amphibians. Bioscience 50:653–666
Grant BW, Dunham AE (1990) Elevational covariation in environmental constraints and life histories of the desert lizard Sceloporus merriami. Ecology 71:1765–1776
Guo XG, Wang YZ (2007) Partitioned Bayesian analyses, dispersal-vicariance analysis, and the biogeography of Chinese toad-headed lizards (Agamidae: Phrynocephalus): a re-evaluation. Mol Phylogenet Evol 45:643–662
Huey RB (1982) Temperature, physiology, and the ecology of reptiles. In: Gans C, Pough FH (eds) Biology of reptilia, vol 13. Academic, London, pp 25–91
Huey RB, Tewksbury JJ (2009) Can behavior douse the fire of climate warming? Proc Natl Acad Sci USA 106:3647–3648
Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Pérez HJÁ, Garland T Jr (2009) Why tropical forest lizards are vulnerable to climate warming. Proc R Soc B 276:1939–1948
Huey RB, Losos JB, Moritz C (2010) Are lizards toast? Science 328:832–833
Hutchison VH (1976) Factors influencing thermal tolerances of individual organisms. In: Esch GW, McFarlane RW (eds) Thermal ecology 11. Proceedings of the 2nd SREL thermal ecology symposium. US National Technical Information Service, Oak Ridge, TN, pp 10–26
Ji X, Lin LH, Luo LG, Lu HL, Gao JF, Han J (2006) Gestation temperature affects sexual phenotype, morphology, locomotor performance and growth of neonatal brown forest skink, Sphenomorphus indicus. Biol J Linn Soc 88:453–463
Ji X, Lin CX, Lin LH, Qiu QB, Du Y (2007) Evolution of viviparity in warm-climate lizards: an experimental test of the maternal manipulation hypothesis. J Evol Biol 20:1037–1045
Ji X, Wang YZ, Wang Z (2009) New species of Phrynocephalus (Squamata, Agamidae) from Qinghai, Northwest China. Zootaxa 1988:61–68
Kearney M, Shine R, Porter WP (2009) The potential for behavioral thermoregulation to buffer ‘‘cold-blooded’’ animals against climate warming. Proc Natl Acad Sci USA 106:3835–3840
Kubička L, Kratochvíl L (2009) First growth, then breed and finally get fat: hierarchical allocation to life-history traits in a lizard with invariant clutch size. Funct Ecol 23:595–601
Lambert SM, Wiens JJ (2013) Evolution of viviparity: a phylogenetic test of the cold-climate hypothesis in Phrynosomatid lizards. Evolution 67:2614–2630
Li H, Qu YF, Hu RB, Ji X (2009a) Evolution of viviparity in cold-climate lizards: testing the maternal manipulation hypothesis. Evol Ecol 23:777–790
Li H, Wang Z, Mei WB, Ji X (2009b) Temperature acclimation affects thermal preference and tolerance in three Eremias lizards (Lacertidae). Curr Zool 55:258–265
Li H, Zhou ZS, Wu T, Wu YQ, Ji X (2013) Do fluctuations in incubation temperature affect hatchling quality in the Chinese soft-shelled turtle Pelodiscus sinensis? Aquaculture 406–407:91–96
Lucas A (1996) Bioenergetics of aquatic animals. Taylor and Francis Ltd, London
Luo LG, Ding GH, Ji X (2010) Income breeding and temperature-induced plasticity in reproductive traits in lizards. J Exp Biol 213:2073–2078
Lynch VJ (2009) Live-birth in vipers (Viperidae) is a key innovation and adaptation to global cooling during the Cenozoic. Evolution 63:2457–2465
Martin TL, Huey RB (2008) Why suboptimal is optimal: Jensen’s inequality and ectotherm thermal preferences. Am Nat 171:E102–E118
Mathies T, Andrews RM (1997) Influence of pregnancy on thermal biology of the lizard, Sceloporus jarrovi: why do pregnant females exhibit low body temperatures? Funct Ecol 11:498–507
McNab BK (2002) The physiological ecology of vertebrates: a view from energetics, vol 1. Comstock, Cornell
Miles DB, Sinervo B, Frankino WA (2000) Reproductive burden, locomotor performance, and the cost of reproduction in free ranging lizards. Evolution 54:1386–1395
Nagy KA (1983) Ecological energetics. In: Huey RB, Pianka ER, Schoener TW (eds) Lizard ecology: studies of a model organism. Harvard University Press, Cambridge, pp 24–54
Noble D, Qi Y, Fu JZ (2010) Species delineation using Bayesian model-based assignment tests: a case study using Chinese toad-headed agamas (genus Phrynocephalus). BMC Evol Biol 10:197
Pang JF, Wang YZ, Zhong Y, Hoelzel AR, Papenfuss TJ, Zeng XM, Ananjeva NB, Zhang YP (2003) A phylogeny of Chinese species in the genus Phrynocephalus (Agamidae) inferred from mitochondrial DNA sequences. Mol Phylogenet Evol 27:398–409
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Patterson JW (1991) Emergence, basking behaviour, mean selected temperature and critical thermal minimum in high and low altitude subspecies of the tropical lizard Mabuya striata. Afr J Ecol 37:330–339
Pincheira-Donoso D, Tregenza T, Witt MJ, Hodgson DJ (2013) The evolution of viviparity opens opportunities for lizard radiation but drives it into a climatic cul-de-sac. Global Ecol Biogeogr 22:857–867
Porter WP, Tracy CR (1983) Biophysical analyses of energetics, time-space utilization, and distributional limits. In: Huey RB, Pianka ER, Schoener TW (eds) Lizard ecology: studies of a model organism. Harvard University Press, Cambridge, pp 55–83
Post E, Pedersen C, Wilmers CC, Forchhammer MC (2008) Warming, plant phenology and the spatial dimension of trophic mismatch for large herbivores. Proc R Soc B 275:2005–2013
Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sánchez-Azofeifa GA, Still CJ, Young BE (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161–167
Pyron RA, Burbrink FT (2014) Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecol Lett 17:13–21
Qu YF, Li H, Gao JF, Ji X (2011a) Embryonic thermosensitivity and hatchling morphology differ between two coexisting lizards. Acta Oecol 37:375–380
Qu YF, Li H, Gao JF, Xu XF, Ji X (2011b) Thermal preference, thermal tolerance and the thermal dependence of digestive performance in two coexisting Phrynocephalus lizards (Agamidae), with a review of species studied. Curr Zool 57:684–700
Qualls CP, Andrews RM (1999) Maternal body volume constrains water uptake by lizard eggs in utero. Funct Ecol 13:845–851
Qualls CP, Shine R (1995) Maternal body-volume as a constraint on reproductive output in lizards: evidence from the evolution of viviparity. Oecologia 103:73–78
Shine R (1985) The evolution of viviparity in reptiles: an ecological analysis. In: Gans C, Billet F (eds) Biology of the reptilia, vol 15. Wiley, New York, pp 605–694
Shine R (1992) Relative clutch mass and body shape in lizards and snakes: is reproductive investment constrained or optimized? Evolution 46:828–833
Shu L, Zhang QL, Qu YF, Ji X (2010) Thermal tolerance, selected body temperature and thermal dependence of food assimilation and locomotor performance in the Qinghai toad-headed lizard Phrynocephalus vlanglii. Acta Ecol Sin 30:2036–2042
Sinervo B, Méndez-de-la-Cruz F, Miles DB, Heulin B, Bastiaans E, Cruz MVS, Lara-Resendiz R, Martínez-Méndez N, Calderón-Espinosa ML, Meza-Lázaro RN, Gadsden H, Avila LJ, Morando M, De la Riva IJ, Sepulveda PV, Rocha CFD, Ibargüengoytía N, Puntriano CA, Massot M, Lepetz V, Oksanen TA, Chapple DG, Bauer AM, Branch WR, Clobert J, Sites JW Jr (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Tang XL, Yue F, Ma M, Wang NB, He JZ, Chen Q (2012) Effects of thermal and hydric conditions on egg incubation and hatchling phenotypes in two Phrynocephalus lizards. Asian Herpetol Res 3:184–191
Telemeco RS, Elphick MJ, Shine R (2009) Nesting lizards (Bassiana duperreyi) compensate partly, but not completely, for climate change. Ecology 90:17–21
Tewksbury JJ, Huey RB, Deutsch CA (2008) Putting the heat on tropical animals. Science 320:1296–1297
Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, Ferreira de Siqueira M, Grainger A, Hannah L, Hughes L, Huntley B, van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Peterson AT, Phillips OL, Williams SE (2004) Extinction risk from climate change. Nature 427:145–148
Tinkle DW, Gibbons JW (1977) The distribution and evolution of viviparity in reptiles. Misc Publ Univ Mich Mus Zool 154:1–55
Urban MC, Richardson JL, Freidenfelds NA (2014) Plasticity and genetic adaptation mediate amphibian and reptile responses to climate change. Evol Appl 7:88–103
Visser ME, Both C, Lambrechts MM (2004) Global climate change leads to mistimed avian reproduction. Adv Ecol Res 35:89–110
Wang YZ, Zeng XM, Fang ZL, Wu GF, Liu ZJ, Papenfuss TJ, Macey JR (2002) A valid species of the genus Phrynocephalus: P. putjatia and a discussion on taxonomy of Phrynocephalus hongyuanensis (Sauria: Agamidae). Acta Zootax Sin 27:372–383
Wang Z, Lu HL, Ma L, Ji X (2013a) Differences in thermal preference and tolerance among three Phrynocephalus lizards (Agamidae) with different body sizes and habitat use. Asian Herpetol Res 4:214–220
Wang Z, Ma L, Shao M, Ji X (2013b) Differences in incubation length and hatchling morphology among five oviparous Phrynocephalus lizards (Agamidae) from China. Asian Herpetol Res 4:225–232
Wang Z, Lu HL, Ma L, Ji X (2014) Viviparity in high-altitude Phrynocephalus lizards is adaptive because embryos cannot fully develop without maternal thermoregulation. Oecologia 174:639–649
Watson CM, Makowsky R, Bagley JC (2014) Reproductive mode evolution in lizards revisited: updated analyses examining geographic, climatic and phylogenetic effects support the cold-climate hypothesis. J Evol Biol 27:2767–2780
Yang J, Sun YY, An H, Ji X (2008) Northern grass lizards (Takydromus septentrionalis) from different populations do not differ in thermal preference and thermal tolerance when acclimated under identical thermal conditions. J Comp Physiol B 178:343–349
Zhao KT (1999) Phrynocephalus przewalskii Strauch, 1876. In: Zhao EM, Zhao KT, Zhou KY (eds) Fauna Sinica, Reptilia, vol 2. Science, Beijing, pp 182–184
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (Projects 31672277, 31470471, 31200282 and 31071910) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We thank Ce Chen, Ya-Qing Liu, and Zhu-Yuan Zhang for help during this research.
Author information
Authors and Affiliations
Contributions
ZW and XJ conceived and designed the experiments. ZW, LM, and MS performed the experiments. ZW, LM, and XJ analyzed the data. ZW and XJ wrote the manuscript; LM and MS provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All experimental procedures complied with the current laws on animal welfare and research in China, and were approved by the Animal Research Ethics Committee of Nanjing Normal University (AREC2010-05-006).
Additional information
Communicated by Raoul Van Damme.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Z., Ma, L., Shao, M. et al. Are viviparous lizards more vulnerable to climate warming because they have evolved reduced body temperature and heat tolerance?. Oecologia 185, 573–582 (2017). https://doi.org/10.1007/s00442-017-3979-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3979-0