Abstract
Purpose
To compare the chromatic pupillary light responses (PLR) in healthy subjects with those from patients with diseases of the outer or inner retina under various stimulus conditions, and to ascertain the parameters required to optimally distinguish between disease and control groups.
Methods
Fifteen patients with retinitis pigmentosa (RP), 19 patients with optic nerve disease (ON), and 16 healthy subjects were enrolled in this prospective study. ON included optic neuritis (NNO) and non-arteritic anterior ischemic optic neuropathy (NAION). For each subject, the PLR was recorded, to red, yellow, green, and blue stimuli for durations of 4 and 12 s, and for stimulus intensities of 4 lx and 28 lx.
Results
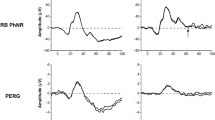
Comparison between control and RP or ON patient results showed that responses after stimulus onset were significantly different for most stimulus conditions, but the post-stimulus amplitudes at 3 s and 7 s after light extinction were not. On the other hand, the difference between the ON and RP groups was significant only for post-stimuli time-points and only for blue stimuli. Differences between responses to blue and red were significantly different, predominantly at post stimulus time-points. A ROC analysis revealed that the maximal constriction amplitudes to a 4 lx, 4 s yellow stimulus are significantly different in ON vs RP patients, and the responses to a 4 s, 28 lx blue stimulus at 7 s post-stimulus are significantly different in controls vs ON vs RP patients with a high specificity.
Conclusions
Pupillary light responses to blue light in healthy, RP, and ON subjects are significantly different from one another. The optimal stimuli for future protocols was found to be a 4 s blue stimulus at 28 lx, and a 4 s yellow stimulus at 4 lx.






Similar content being viewed by others
References
Hattar S, Liao HW, Takao M, Berson DM, Yau KW (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295:1065–1070
Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM (2006) Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 497(3):326–349. doi:10.1002/cne.20970
Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM (2007) Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vis Res 47(7):946–954. doi:10.1016/j.visres.2006.12.015
Provencio I, Cooper HM, Foster RG (1998) Retinal projections in mice with inherited retinal degeneration: implications for circadian photoentrainment. J Comp Neurol 395(4):417–439
Hannibal J, Fahrenkrug J (2002) Melanopsin: a novel photopigment involved in the photoentrainment of the brain’s biological clock? Ann Med 34(5):401–407
Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD (1998) Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A 95(1):340–345
Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE (2003) Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol 460(3):380–393. doi:10.1002/cne.10652
Berson DM, Dunn FA, Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073
Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW (2005) Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433(7027):741–745. doi:10.1038/nature03344
Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM (2005) Induction of photosensitivity by heterologous expression of melanopsin. Nature 433(7027):745–749. doi:10.1038/nature03345
Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD (2005) Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433(7027):749–754
Gamlin PD (2006) The pretectum: connections and oculomotor-related roles. Prog Brain Res 151:379–405. doi:10.1016/S0079-6123(05)51012-4
Lucas RJ, Douglas RH, Foster RG (2001) Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci 4(6):621–626. doi:10.1038/88443
Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW (2003) Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424(6944):76–81. doi:10.1038/nature01761
Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A (2009) Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology 116(8):1564–1573. doi:10.1016/j.ophtha.2009.02.007
McDougal DH, Gamlin PD (2010) The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vis Res 50(1):72–87. doi:10.1016/j.visres.2009.10.012
Ishikawa H, Onodera A, Asakawa K, Nakadomari S, Shimizu K (2012) Effects of selective-wavelength block filters on pupillary light reflex under red and blue light stimuli. Jpn J Ophthalmol 56(2):181–186. doi:10.1007/s10384-011-0116-1
Hattar S, Liao HW, Takao M, Berson DM, Yau KW (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295(5557):1065–1070. doi:10.1126/science.1069609
Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW (2003) Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299(5604):245–247. doi:10.1126/science.1077293
Kawasaki A, Kardon RH (2007) Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol 27(3):195–204. doi:10.1097/WNO.0b013e31814b1df9
Young RS, Kimura E (2008) Pupillary correlates of light-evoked melanopsin activity in humans. Vis Res 48(7):862–871. doi:10.1016/j.visres.2007.12.016
Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC (2011) Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci 52(9):6624–6635. doi:10.1167/iovs.11-7586
Kawasaki A, Crippa SV, Kardon R, Leon L, Hamel C (2012) Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Invest Ophthalmol Vis Sci 53(9):5562–5569. doi:10.1167/iovs.12-10230
Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A (2011) Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology 118(2):376–381. doi:10.1016/j.ophtha.2010.06.033
Kankipati L, Girkin CA, Gamlin PD (2011) The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci 52(5):2287–2292. doi:10.1167/iovs.10-6023
Leon L, Crippa SV, Borruat FX, Kawasaki A (2012) Differential effect of long versus short wavelength light exposure on pupillary re-dilation in patients with outer retinal disease. Clin Exp Ophthalmol 40(1):e16–e24. doi:10.1111/j.1442-9071.2011.02665.x
Kawasaki A, Collomb S, Leon L, Munch M (2014) Pupil responses derived from outer and inner retinal photoreception are normal in patients with hereditary optic neuropathy. Exp Eye Res 120:161–166. doi:10.1016/j.exer.2013.11.005
Moura AL, Nagy BV, La Morgia C, Barboni P, Oliveira AG, Salomao SR, Berezovsky A, de Moraes-Filho MN, Chicani CF, Belfort R Jr, Carelli V, Sadun AA, Hood DC, Ventura DF (2013) The pupil light reflex in Leber’s hereditary optic neuropathy: evidence for preservation of melanopsin-expressing retinal ganglion cells. Invest Ophthalmol Vis Sci 54(7):4471–4477. doi:10.1167/iovs.12-11137
Kankipati L, Girkin CA, Gamlin PD (2010) Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci 51(5):2764–2769. doi:10.1167/iovs.09-4717
Mure LS, Cornut PL, Rieux C, Drouyer E, Denis P, Gronfier C, Cooper HM (2009) Melanopsin bistability: a fly’s eye technology in the human retina. PLoS ONE 4(6):e5991. doi:10.1371/journal.pone.0005991
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J (2009) Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet 374(9701):1597–1605. doi:10.1016/S0140-6736(09)61836-5
Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP, Gekeler F, Greppmaier U, Harscher A, Kibbel S, Koch J, Kusnyerik A, Peters T, Stingl K, Sachs H, Stett A, Szurman P, Wilhelm B, Wilke R (2011) Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc R Soc B 278(1711):1489–1497. doi:10.1098/rspb.2010.1747
Acknowledgments
We thank W Durst for his help with the calibration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding and grants
None.
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Richter, P., Wilhelm, H., Peters, T. et al. The diagnostic accuracy of chromatic pupillary light responses in diseases of the outer and inner retina. Graefes Arch Clin Exp Ophthalmol 255, 519–527 (2017). https://doi.org/10.1007/s00417-016-3496-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3496-6