Abstract
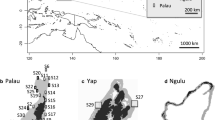
Bleaching events are becoming more frequent and are projected to become annual in Micronesia by 2040. To prepare for this threat, the Government of Palau is reviewing its marine protected area network to increase the resilience of the reefs by integrating connectivity into the network design. To support their effort, we used high-throughput sequencing of microsatellites to create genotypes of colonies of the coral Acropora hyacinthus to characterize population genetic structure and dispersal patterns that led to the recovery of Palau’s reefs from a 1998 bleaching event. We found no evidence of a founder effect or refugium where colonies may have survived to recolonize the reef. Instead, we found significant pairwise F′ st values, indicating population structure and low connectivity among most of the 25 sites around Palau. We used kinship to measure genetic differences at the individual level among sites and found that differences were best explained by the degree of exposure to the ocean [F 1,20 = 3.015, Pr(>F) = 0.01], but with little of the total variation explained. A permutation test of the pairwise kinship coefficients revealed that there was self-seeding within sites. Overall, the data point to the population of A. hyacinthus in Palau recovering from a handful of surviving colonies with population growth primarily from self-seeding and little exchange among sites. This finding has significant implications for the management strategies for the reefs of Palau, and we recommend increasing the number and distribution of management areas around Palau to capture the genetic architecture and increase the chances of protecting potential refuges in the future.



Similar content being viewed by others
References
Australian Bureau of Meteorology and CSIRO (2014) Climate variability, extremes and change in the western Tropical Pacific: new science and updated country reports. Pacific-Australia Climate Change Science and Adaptation Planning Program technical report, Australian Bureau of Meteorology and Commonwealth Scientific and Industrial Research Organisation, Melbourne, Australia
Almany GR, Connolly SR, Heath DD, Hogan JD, Jones GP, McCook LJ, Mills M, Pressey RL, Williamson DH (2009) Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs 28:339–351
Andréfouët S, Berkelmans R, Odriozola L, Done T, Oliver J, Müller-Karger F (2002) Choosing the appropriate spatial resolution for monitoring coral bleaching events using remote sensing. Coral Reefs 21:147–154
Andutta FP, Kingsford MJ, Wolanski E (2012) “Sticky water” enables the retention of larvae in a reef mosaic. Estuar Coast Shelf Sci 101:54–63
Ayre DJ, Hughes TP (2000) Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54:1590–1605
Bahr KD, Jokiel PL, Toonen RJ (2015) The unnatural history of Kāne’ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ 3:e950
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Baums IB, Paris CB, Chérubin LM (2006) A bio-oceanographic filter to larval dispersal in a reef-building coral. Limnol Oceanogr 51:1969–1981
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Benestan L, Ferchaud A-L, Hohenlohe P, Garner BA, Naylor GJP, Baums I, Schwartz M, Kelley JL, Luikart G (2016) Conservation genomics of natural and managed populations: building a conceptual and practical framework. Mol Ecol 25:2967–2977
Bernardi G, Beldade R, Holbrook SJ, Schmitt RJ (2012) Full-sibs in cohorts of newly settled coral reef fishes. PLoS One 7:e44953
Bruno J, Siddon C, Witman J, Colin P, Toscano M (2001) El Niño related coral bleaching in Palau, Western Caroline Islands. Coral Reefs 20:127–136
Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. World Resources Institute, Washington
Chollett I, Mumby PJ (2013) Reefs of last resort: locating and assessing thermal refugia in the wider Caribbean. Biol Conserv 167:179–186
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Ann Rev Mar Sci 1:443–466
Cros A, Toonen RJ, Davies SW, Karl SA (2016) Population genetic structure between Yap and Palau for the coral Acropora hyacinthus. PeerJ 4:e2330
D’Aloia CC, Bogdanowicz SM, Majoris JE, Harrison RG, Buston PM (2013) Self-recruitment in a Caribbean reef fish: a method for approximating dispersal kernels accounting for seascape. Mol Ecol 22:2563–2572
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Doropoulos C, Roff G, Zupan M, Nestor V, Isechal AL, Mumby PJ (2014) Reef-scale failure of coral settlement following typhoon disturbance and macroalgal bloom in Palau, Western Pacific. Coral Reefs 33:613–623
Eldon B, Riquet F, Yearsley J, Jollivet D, Broquet T (2016) Current hypotheses to explain genetic chaos under the sea. Curr Zool 62:551–566
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Fernandes L, Day J, Lewis A, Slegers S, Kerrigan B, Breen D, Cameron D, Jago B, Hall J, Lowe D, Innes J, Tanzer J, Chadwick V, Thompson L, Gorman K, Simmons M, Barnett B, Sampson K, De’ath G, Mapstone B, Marsh H, Possingham H, Ball I, Ward T, Dobbs K, Aumend J, Slater D, Stapleton K (2005) Establishing representative no-take areas in the Great Barrier Reef: large-scale implementation of theory on marine protected areas. Conserv Biol 19:1733–1744
Foster NL, Paris CB, Kool JT, Baums IB, Stevens JR, Sanchez JA, Bastidas C, Agudelo C, Bush P, Day O, Ferrari R, Gonzalez P, Gore S, Guppy R, McCartney MA, McCoy C, Mendes J, Srinivasan A, Steiner S, Vermeij MJA, Weil E, Mumby PJ (2012) Connectivity of Caribbean coral populations: complementary insights from empirical and modelled gene flow. Mol Ecol 21:1143–1157
Gaither MR, Szabo Z, Crepeau MW, Bird CE, Toonen RJ (2011) Preservation of corals in salt-saturated DMSO buffer is superior to ethanol for PCR experiments. Coral Reefs 30:329–333
Gaither MR, Bowen BW, Toonen RJ, Serge P, Vanessa M, John E, Ross Robertson D (2010) Genetic consequences of introducing allopatric lineages of bluestripe snapper (Lutjanus kasmira) to Hawaii. Mol Ecol 19:1107–1121
Golbuu Y (2011) Responses of Palau’s coral reefs to disturbances at multiple scales. PhD thesis, Southern Cross University, Lismore, Australia
Golbuu Y, Wolanski E, Harrison P, Richmond RH, Victor S, Fabricius KE (2011) Effects of land-use change on characteristics and dynamics of watershed discharges in Babeldaob, Palau, Micronesia. J Mar Biol 2011:981273
Golbuu Y, Victor S, Penland L, Idip D, Emaurois C, Okaji K, Yukihira H, Iwase A, van Woesik R (2007) Palau’s coral reefs show differential habitat recovery following the 1998 bleaching event. Coral Reefs 26:319–332
Golbuu Y, Wolanski E, Idechong JW, Victor S, Isechal AL, Oldiais NW, Idip D, Richmond RH, van Woesik R (2012) Predicting coral recruitment in Palau’s complex reef archipelago. PLoS One 7:e50998
Gorospe KD, Karl SA (2015) Depth as an organizing force in Pocillopora damicornis: intra-reef genetic architecture. PLoS One 10:e0122127
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Hellberg ME (2007) Footprints on water: the genetic wake of dispersal among reefs. Coral Reefs 26:463–473
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (2000) Supply-side ecology works both ways: the link between benthic adults, fecundity, and larval recruits. Ecology 81:2241–2249
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nyström M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Iacchei M, Ben-Horin T, Selkoe KA, Bird CE, García-Rodríguez FJ, Toonen RJ (2013) Combined analyses of kinship and FST suggest potential drivers of chaotic genetic patchiness in high gene-flow populations. Mol Ecol 22:3476–3494
IUCN (2008) Establishing resilient marine protected area networks—making it happen. The World Conservation Union (IUCN), Washington, DC
Jones G, Srinivasan M, Almany G (2007) Population connectivity and conservation of marine biodiversity. Oceanography 20:100–111
Jones GP, Almany GR, Russ GR, Sale PF, Steneck RS, Van Oppen MJH, Willis BL (2009) Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28:307–325
Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I (2015) Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Mol Ecol Resour 15:1179–1191
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Ladner JT, Palumbi SR (2012) Extensive sympatry, cryptic diversity and introgression throughout the geographic distribution of two coral species complexes. Mol Ecol 21:2224–2238
Lagabrielle E, Crochelet E, Andrello M, Schill SR, Arnaud-Haond S, Alloncle N, Ponge B (2014) Connecting MPAs—eight challenges for science and management. Aquat Conserv 24:94–110
Levin LA (2006) Recent progress in understanding larval dispersal: new directions and digressions. Integr Comp Biol 46:282–297
Lewis SM (1986) The role of herbivorous fishes in the organization of a Caribbean reef community. Ecol Monogr 56:183–200
Liggins L, Treml EA, Riginos C (2013) Taking the plunge: an introduction to undertaking seascape genetic studies and using biophysical models. Geography Compass 7:173–196
Luikart G, Allendorf FW, Cornuet JM, Sherwin WB (1998) Distortion of allele frequency distributions provides a test for recent population bottlenecks. J Hered 89:238–247
Magris RA, Pressey RL, Weeks R, Ban NC (2014) Integrating connectivity and climate change into marine conservation planning. Biol Conserv 170:207–221
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Márquez LM, van Oppen MJH, Willis BL, Miller DJ (2002) Sympatric populations of the highly cross-fertile coral species Acropora hyacinthus and Acropora cytherea are genetically distinct. Proc R Soc Lond B Biol Sci 269:1289–1294
McClanahan TR, Muthiga NA, Coleman RA (2011) Testing for top-down control: can post-disturbance fisheries closures reverse algal dominance? Aquat Conserv 21:658–675
McLeod E, Salm R, Green A, Almany J (2009) Designing marine protected area networks to address the impacts of climate change. Front Ecol Environ 7:362–370
Meirmans PG, van Tienderen PH (2004) GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
Mumby PJ, Harborne AR, Williams J, Kappel CV, Brumbaugh DR, Micheli F, Holmes KE, Dahlgren CP, Paris CB, Blackwell PG (2007) Trophic cascade facilitates coral recruitment in a marine reserve. Proc Natl Acad Sci U S A 104:8362–8367
Mumby PJ, Elliott IA, Eakin CM, Skirving W, Paris CB, Edwards HJ, Enríquez S, Iglesias-Prieto R, Cherubin LM, Stevens JR (2011) Reserve design for uncertain responses of coral reefs to climate change. Ecol Lett 14:132–140
Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30:429–440
Pinsky ML, Palumbi SR, Andréfouët S, Purkis SJ (2012) Open and closed seascapes: where does habitat patchiness create populations with high fractions of self-recruitment? Ecol Appl 22:1257–1267
Piry S, Luikart G, Cornuet JM (1999) BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90:502–503
Pringle JM, Wares JP (2007) Going against the flow: maintenance of alongshore variation in allele frequency in a coastal ocean. Mar Ecol Prog Ser 335:69–84
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rice P, Longden I, Bleasby A (2000) EMBOSS: the European molecular biology open software suite. Trends Genet 16:276–277
Riginos C, Liggins L (2013) Seascape genetics: populations, individuals, and genes marooned and adrift. Geography Compass 7:197–216
Salm RV, Done T, McLeod E (2006) Marine protected area planning in a changing climate. In: Phinney JT, Hoegh-Guldberg O, Kleypas J, Skirving W, Strong A (eds) Coral reefs and climate change: science and management. American Geophysical Union, Washington, D. C., pp 207–222
Selig ER, Casey KS, Bruno JF (2012) Temperature-driven coral decline: the role of marine protected areas. Glob Chang Biol 18:1561–1570
Selkoe KA, Henzler CM, Gaines SD (2008) Seascape genetics and the spatial ecology of marine populations. Fish Fish 9:363–377
Selkoe KA, Gaines SD, Caselle JE, Warner RR (2006) Current shifts and kin aggregation explain genetic patchiness in fish recruits. Ecology 87:3082–3094
Selkoe AKA, Aloia CCD, Crandall ED, Iacchei M (2016) A decade of seascape genetics: contributions to basic and applied marine connectivity. Mar Ecol Prog Ser 554:1–19
Shanks AL (2009) Pelagic larval duration and dispersal distance revisited. Biol Bull 216:373–385
Steneck RS, Paris CB, Arnold SN, Ablan-Lagman MC, Alcala AC, Butler MJ, McCook LJ, Russ GR, Sale PF (2009) Thinking and managing outside the box: coalescing connectivity networks to build region-wide resilience in coral reef ecosystems. Coral Reefs 28:367–378
Thompson DM, van Woesik R (2009) Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc R Soc Lond B Biol Sci 276:2893–2901
Toh TC, Guest JR, Chou LM (2012) Coral larval rearing in Singapore: observations on spawning timing, larval development and settlement of two common scleractinian coral species. Contributions to Marine Science, National University of Singapore, Republic of Singapore, pp 81–87
Toh TC, Peh JWK, Chou LM (2013) Early onset of zooplanktivory in equatorial reef coral recruits. Mar Biodivers 43:177–178
Toonen RJ, Grosberg RK (2011) Causes of chaos: spatial and temporal genetic heterogeneity in the intertidal anomuran crab Petrolisthes cinctipes. In: Koenemann S, Held C, Schubart C (eds) Phylogeography and population genetics in Crustacea. Crustacean issues series. CRC Press, Boca Raton, pp 75–107
van Hooidonk R, Maynard JA, Planes S (2013) Temporary refugia for coral reefs in a warming world. Nat Clim Chang 3:508–511
Victor S, Golbuu Y, Yukihira H, Van Woesik R (2009) Acropora size-frequency distributions reflect spatially variable conditions on coral reefs of Palau. Bull Mar Sci 85:149–157
Wallace CC (1985) Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus Acropora. Mar Biol 88:217–233
White C, Selkoe KA, Watson J, Siegel DA, Zacherl DC, Toonen RJ (2010) Ocean currents help explain population genetic structure. Proc R Soc Lond B Biol Sci 277:1685–1694
Wolanski E (1994) Physical oceanographic processes of the Great Barrier Reef. CRC Press, Boca Raton
Wolanski E, Spagnol S (2000) Sticky waters in the Great Barrier Reef. Estuar Coast Shelf Sci 50:27–32
Acknowledgements
We are grateful to M Belcaid and Y Cros for assistance with raw data processing and to the following University of Hawai‘i undergraduates for help in the laboratory: I Buffenstein, G Ciszek, B Haun, K Kaneshiro, M Keliipuleole, H Lim, K Niimoto, A Sifrit and T Whitman. We also thank M Iacchei for help with the manuscript and K Edwards for help with the analysis. Special thanks to The Nature Conservancy and Palau International Coral Reef Center for enabling the fieldwork and shipping permits. All collections were done under CITES permit PW 12-091 and a Palau Marine Research Permit RE-12-27. Funding was provided to A Cros and SA Karl by the Disney Wildlife Conservation Fund, to A Cros by the Graduate Women in Science Adel Lewis Grant Fellowship, the Founder Region Fellowship, the Ecology Evolution Conservation Biology Watson T. Yoshimoto grant and the Colonel Willys E. Lord Scholarship Award and the National Science Foundation grant OCE 12-60169 to RJ Toonen. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We also thank the HIMB NSF-EPSCoR Core Genetics Lab Facility (NSF, EPS-0903833). This is the Hawai‘i Institute of Marine Biology contribution # 1677 and the School for Ocean and Earth Science and Technology contribution # 9906.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Line K. Bay
Electronic supplementary material
Below is the link to the electronic supplementary material.
338_2017_1565_MOESM1_ESM.pdf
Fig. S1 clumpak summary plots of Bayesian clustering algorithm of the genotypes of colonies at 25 sites in Palau implemented in structure with a no-admixture model with location as a prior for K = 2 to 4 (PDF 22268 kb)
Rights and permissions
About this article
Cite this article
Cros, A., Toonen, R.J., Donahue, M.J. et al. Connecting Palau’s marine protected areas: a population genetic approach to conservation. Coral Reefs 36, 735–748 (2017). https://doi.org/10.1007/s00338-017-1565-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-017-1565-x