Abstract
Key message
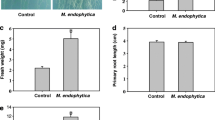
Endophytic microbes Bacillus sp. LZR216 isolated from Arabidopsis root promoted Arabidopsis seedlings growth. It may be achieved by promoting the lateral root growth and inhibiting the primary root elongation.
Abstract
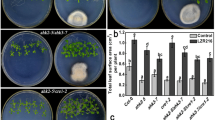
Plant roots are colonized by an immense number of microbes, including epiphytic and endophytic microbes. It was found that they have the ability to promote plant growth and protect roots from biotic and abiotic stresses. But little is known about the mechanism of the endophytic microbes-regulated root development. We isolated and identified a Bacillus sp., named as LZR216, of endophytic bacteria from Arabidopsis root. By employing a sterile experimental system, we found that LZR216 promoted the Arabidopsis seedlings growth, which may be achieved by promoting the lateral root growth and inhibiting the primary root elongation. By testing the cell type-specific developmental markers, we demonstrated that Bacillus sp. LZR216 increases the DR5::GUS and DR5::GFP expression but decreases the CYCB1;1::GUS expression in Arabidopsis root tips. Further studies indicated that LZR216 is able to inhibit the meristematic length and decrease the cell division capability but has little effect on the quiescent center function of the root meristem. Subsequently, it was also shown that LZR216 has no significant effects on the primary root length of the pin2 and aux1-7 mutants. Furthermore, LZR216 down-regulates the levels of PIN1-GFP, PIN2-GFP, PIN3-GFP, and AUX1-YFP. In addition, the wild-type Arabidopsis seedlings in the present of 1 or 5 µM NPA (an auxin transport inhibitor) were insensitive to LZR216-inhibited primary root elongation. Collectively, LZR216 regulates the development of root system architecture depending on polar auxin transport. This study shows a new insight on the ability of beneficial endophytic bacteria in regulating postembryonic root development.








Similar content being viewed by others
Abbreviations
- CFU:
-
Colony-forming units
- GFP:
-
Green fluorescent protein
- GUS:
-
β-Glucuronidase
- KB:
-
King’s B medium
- NPA:
-
1-N-Naphthylphthalamic acid
- PAT:
-
Polar auxin transport
- PGPB:
-
Plant growth-promoting bacteria
- PGPR:
-
Plant growth-promoting rhizobacteria
- QC:
-
Quiescent center
- YFP:
-
Yellow fluorescent protein
References
Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100:2992–2997
Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9:26–32
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Barazani O, Friedman J (1999) Is IAA the major root growth factor secreted from plant-growth-mediating bacteria? J Chem Ecol 25:2397–2406
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Berg G, Smalla K (2012) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Bowen GD, Rovira AD (1999) The rhizosphere and its management to improve plant growth. Adv Agron 66:1–102
Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, Bennett M (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13:843–852
Chaiharn M, Chunhaleuchanon S, Lumyong S (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microbiol Biotechnol 25:1919–1928
Combes-Meynet E, Pothier JF, Moënne-Loccoz Y, Prigent-Combaret C (2011) The Pseudomonas secondary metabolite 2, 4-diacetylphloroglucinol is a signal inducing rhizoplane expression of Azospirillum genes involved in plant-growth promotion. Mol Plant Microbe Interact 24:271–284
Contesto C, Milesi S, Mantelin S, Zancarini A, Desbrosses G, Varoquaux F, Bellini C, Kowalczyk M, Touraine B (2010) The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 232:1455–1470
Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149:1579–1592
Couillerot O, Prigent-Combaret C, Caballero-Mellado J, Moënne-Loccoz Y (2009) Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett Appl Microbiol 48:505–512
Dobbelaere S, Croonenborghs A, Thys A, Broek AV, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:153–162
Felten J, Kohler A, Morin E, Bhalerao RP, Palme K, Martin F, Ditengou FA, Legué V (2009) The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol 151:1991–2005
Jing YD, He ZL, Yang XE (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci B 8:192–207
Keller CP, Stahlberg R, Barkawi LS, Cohen JD (2004) Long-term inhibition by auxin of leaf blade expansion in bean and Arabidopsis. Plant Physiol 134:1217–1226
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885–886
Kloepper JW, Lifshitz R, Zablotowicz RM (1989) Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol 7:39–44
López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin-and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Interact 20:207–217
Lucas JA, García-Cristobal J, Bonilla A, Ramos B, Gutierrez-Mañero J (2014) Beneficial rhizobacteria from rice rhizosphere confers high protection against biotic and abiotic stress inducing systemic resistance in rice seedlings. Plant Physiol Biochem 82:44–53
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Persello-Cartieaux F, Nussaume L, Robaglia C (2003) Tales from the underground: molecular plant-rhizobacteria interactions. Plant Cell Environ 26:189–199
Pitts RJ, Cernac A, Estelle M (1998) Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J 16:553–560
Pozo MJ, Azcón-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398
Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S (2002) Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 130:1908–1917
Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50:514–528
Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118:1369–1378
Richardson A, Barea J-M, McNeill A, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932
Ryu C-M, Hu C-H, Locy R, Kloepper J (2005) Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil 268:285–292
Tak H, Ahmad F, Babalola O (2013) Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Rev Environ Contam Toxicol 223:33–52
Tinker PB (1984) The role of microorganisms in mediating and facilitating the uptake of plant nutrients from soil. Plant Soil 76:77–91
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Walker V, Couillerot O, Von Felten A, Bellvert F, Jansa J, Maurhofer M, Bally R, Moënne-Loccoz Y, Comte G (2012) Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil 356:151–163
Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse C (2013) Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol 162:304–318
Zhang H, Kim M-S, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu C-M, Allen R, Melo IS (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31170225; 31201145), Foundation of Science and Technology Program of Gansu Province (1208RJZA224), Foundation of Science and Technology Program of Gansu Province (1107RJYA005), the National High Technology Research and Development Program (2007AA021401), National Program on Key Basic Research Project (2012CB026105), and Fundamental Research Funds for the Central Universities (lzujbky-2012-104).
Conflict of interest
The content in this paper is new. It is not being submitted to any other journal. All the authors listed have approved the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Chong.
J. Wang and Y. Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Zhang, Y., Li, Y. et al. Endophytic microbes Bacillus sp. LZR216-regulated root development is dependent on polar auxin transport in Arabidopsis seedlings. Plant Cell Rep 34, 1075–1087 (2015). https://doi.org/10.1007/s00299-015-1766-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1766-0