Abstract
Purpose
A phase I dose escalation study was performed to determine the maximum tolerated dose (MTD) of intercalated dosing of BMS-690514, a reversible oral panHER/VEGF receptor inhibitor, combined with paclitaxel/carboplatin (PC) in advanced solid tumors. Secondary endpoints included safety, pharmacokinetics (PK), exploratory pharmacodynamics (PD), and preliminary efficacy.
Experimental design
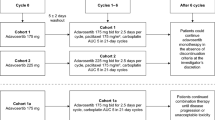
Patients received fixed doses of P (200 mg/m2) and C (AUC 6 mg/mL min) q21 days with intercalated BMS-690514 (Days 4–19) starting at 100 mg/day and increasing by 50 mg/day using a 3 + 3 dose escalation design until the MTD was reached. Twenty additional patients were enrolled in the expansion cohort at the recommended phase II dose (RP2D).
Results
The MTD was reached at 150 mg/day. DLTs included grade 3 thrombosis at 100 mg (1 patient) and grade 3 diarrhea at 150 mg (1 patient) and 200 mg (2 patients). Serious adverse events (AEs) occurring in 20/37 patients included neutropenia (n = 5), diarrhea (n = 4), pulmonary embolism (n = 3), and simultaneous dehydration, acute renal failure, and febrile neutropenia (n = 2). BMS-690514-related AEs included diarrhea (97 %), acneiform rash (60 %), fatigue (43 %), nausea (30 %), and anorexia (30 %). There were no treatment-related deaths. Sequential intermittent administration of PC did not affect the PK of BMS-690514. Of the 32 patients evaluable for efficacy, there were 12 partial responses including five patients with non-small-cell lung cancer and 12 patients with stable disease.
Conclusions
The MTD of intercalated BMS-609514 combined with PC was 150 mg/day. This approach was tolerable with manageable toxicities and antitumor activity in a variety of solid tumor types.


Similar content being viewed by others
Notes
Not for reasons of tolerability or toxicity.
References
Schwartz GN, Pendyala L, Kindler H, Meropol N, Perez R, Raghavan D et al (1998) The clinical development of paclitaxel and the paclitaxel/carboplatin combination. Eur J Cancer 34:1543–1548
Chu Q, Vincent M, Logan D, Mackay JA, Evans WK (2005) Taxanes as first-line therapy for advanced non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer (Amsterdam, Netherlands) 50:355–374
Ozols RF (2002) Update on the management of ovarian cancer. Cancer J (Sudbury, Mass) 8(Suppl 1):S22–S30
Marathe P, Tang Y, Sleczka B, Rodrigues D, Gavai A, Wong T et al (2010) Preclinical pharmacokinetics and in vitro metabolism of BMS-690514, a potent inhibitor of EGFR and VEGFR2. J Pharm Sci 99:3579–3593
Seymour L (2003) Epidermal growth factor receptor inhibitors: an update on their development as cancer therapeutics. Curr Opin Investig Drugs 4:658–666
Chen P, Wang L, Liu B, Zhang HZ, Liu HC, Zou Z (2011) EGFR-targeted therapies combined with chemotherapy for treating advanced non-small-cell lung cancer: a meta-analysis. Eur J Clin Pharmacol 67:235–243
Tsai CM, Chen JT, Stewart DJ, Chiu CH, Lai CL, Hsiao SY et al (2011) Antagonism between gefitinib and cisplatin in non-small cell lung cancer cells: why randomized trials failed? J Thorac Oncol 6:559–568
Gatzmeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F et al (2007) Phase III trial of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 25:1545–1552
Giaccone G, Herbst RS, Manegold C, Kaukel E, Roubec J, De Rosa F et al (2004) Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial - INTACT1. J Clin Oncol 22:777–784
Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C et al (2004) Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial - INTACT2. J Clin Oncol 22:785–794
Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A et al (2005) TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23:5892–5899
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Margolin K (2002) Inhibition of vascular endothelial growth factor in the treatment of solid tumors. Curr Oncol Rep 4:20–28
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V et al (2010) Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 21:1804–1809
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Van Cutsem E, Bajetta E, Valle J, Köhne CH, Hecht JR, Moore M et al (2011) Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol 29:2004–2010
Lee CB, Socinski MA (2007) Vascular endothelial growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: a review of recent clinical trials. Rev Recent Clin Trials 2:117–120
Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D et al (2010) Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC Clinical Trials Group BR24 study. J Clin Oncol 28:49–55
Blumenschein GR, Kabbinavar F, Menon H, Mok TS, Stephenson J, Beck JT et al (2011) A phase II, multicenter, open-label randomized study of motesanib or bevacizumab in combination with paclitaxel and carboplatin for advanced nonsquamous non-small-cell lung cancer. Ann Oncol 22:2057–2067
Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M et al (2010) Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 28:1835–1842
Davies AM, Ho C, Beckett L, Lau D, Scudder SA, Lara PN et al (2009) Intermittent erlotinib in combination with pemetrexed: phase I schedules designed to achieve pharmacodynamic separation. J Thorac Oncol 4:862–868
Sangha R, Davies AM, Lara PN, Mack PC, Beckett LA, Hesketh PJ et al (2001) Intercalated erlotinib-docetaxel dosing schedules designed to achieve pharmacodynamic separation: results of a phase I/II trial. J Thorac Oncol 6:2112–2119
Wong TW, Lee FY, Emanuel S, Fairchild C, Fargnoli J, Fink B et al (2011) Antitumor and antiangiogenic activities of BMS-690514, an inhibitor of human EGF and VEGF receptor kinase families. Clin Cancer Res 17:4031–4041
Gupta A (2010) ErbB/VEGF Receptor Inhibitor: BMS-690514 Investigator Brochure. Bristol-Myers Squibb Research and Development. Princeton, NJ
Chow LQ, Salvati M, Chow P et al (2010) Using FDG-PET in xenograft models to identify dosing regimen in a Phase 1 trial of intercalated BMS-690514 in combination with paclitaxel/carboplatin (PC) in patients with advanced or metastatic solid tumors (poster presentation 168). Multidisciplinary symposium in thoracic oncology (ASCO/ASTRO/IASLC), Chicago, IL
Bahleda R, Felip E, Herbst RS, Hanna NH, Laurie SA, Shepherd FA et al (2008) Phase I multicenter trial of BMS-690514: safety, pharmacokinetic profile, biological effects, and early clinical evaluation in patients with advanced solid tumors and non-small cell lung cancer. J Clin Oncol 26 (suppl; abstr 2564)
Sequist LV, Bell DW, Lynch TJ, Haber DA (2007) Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol 25:587–595
Loriot Y, Mordant P, Dorvault N, De la motte Rouge T, Bourhis J, Soria JC et al (2010) BMS-690514, a VEGFR and EGFR tyrosine kinase inhibitor, shows anti-tumoural activity on non-small-cell lung cancer xenografts and induces sequence-dependent synergistic effect with radiation. Br J Cancer 103:347–353
Mok TSK, Lee JS, Zhang L, Yu C, Thongprasert S, Ladrera GEI et al (2012) Biomarker analyses and overall survival (OS) from the randomized, placebo-controlled, phase 3, fastact-2 study of intercalated erlotinib with first-line chemotherapy in advanced non-small-cell lung cancer (NSCLC). Ann Oncol 23(Suppl 9):PPIX400–PPIX446
Pena C, Lathia C, Shan M, Escudier B, Bukowski RM (2010) Biomarkers predicting outcome in patients with advanced renal cell carcinoma: results from sorafenib phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin Cancer Res 16:4853–4863
Mao C, Qiu LX, Liao RY, Du FB, Ding H, Yang WC et al (2010) KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung cancer 69:272–278
Langer CJ (2011) Roles of EGFR and KRAS mutations in the treatment of patients with Non-small-cell lung cancer. Pharm Ther 36:263–279
Allen-Auerbach M, Weber WA (2009) Measuring response with FDG-PET: methodological aspects. Oncologist 14:369–377
Weber WA, Figlin R (2007) Monitoring cancer treatment with PET/CT: does it make a difference? J Nucl Med 48(Suppl 1):36S–44S
Prior JO, Montemurro M, Orcurto MV, Michielin O, Luthi F, Benhattar J et al (2009) Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol 27:439–445
Revheim ME, Roe K, Bruland OS, Bach-Gansmo T, Skretting A, Seierstad T (2011) Monitoring the Effect of Targeted Therapies in a Gastrointestinal Stromal Tumor Xenograft Using a Clinical PET/CT. Mol Imaging Biol 13:1234–1240
Funck-Brentano C, Jaillon P (1993) Rate-corrected QT interval: techniques and limitations. Am J Cardiol 72(6):17B–22B
Christopher LJ, Hong H, Vakkalagadda B, Clemens PL, Su H, Roongta V et al (2010) Metabolism and disposition of [14C]BMS-690514 ((3R,4R)-4-amino-1-((4-((3-methoxyphenyl)amino)pyrrolo[2,1-f][1, 2, 4]triazin-5-yl)methyl)-3-piperidinol), an ErbB/VEGFR inhibitor, after oral administration to humans. Drug Metab Dispos 38:2049–2059
Hong HZ, Su H, Ma L, Yao M, Iyer RA, Humphreys WG et al (2011) In vitro characterization of the metabolic pathways and P450 inhibition and induction potential of BMS-690514, an ErbB/VEGFR inhibitor. Drug Metab Dispos 39:1658–1667
Tonkin K, Tritchler D, Tannock I (1985) Criteria of tumor response used in clinical trials of chemotherapy. J Clin Oncol 3:870–875
Glusker P, Recht L, Lane B (2006) Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med 354:980–982 (discussion-2)
Kaneda H, Okamoto I, Satoh T, Nakagawa K (2011) Reversible posterior leukoencephalopathy syndrome and trastuzumab. Invest New Drugs [Epub ahead of print]
Martin G, Bellido L, Cruz JJ (2007) Reversible posterior leukoencephalopathy syndrome induced by sunitinib. J Clin Oncol 25:3559
de La Motte Rouge T, Galluzzi L, Olaussen KA, Zermati Y, Tasdemir E, Robert T et al (2007) A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res 67:6253–6262
Bahleda R, Soria J, Harbison C, Park J, Felip E, Hanna N et al (2009) Tumor regression and pharmacodynamic (PD) biomarker validation in non-small cell lung cancer (NSCLC) patients treated with the Erb/VEGFR inhibitor BMS-690514 [ASCO abstract 8098]. J Clin Oncol 27((15S)):11–12
Acknowledgments
The authors wish to thank all of the participating patients and their families, as well as the global network of investigators, research nurses, study coordinators, and operation staff. This study was sponsored by Bristol-Myers Squibb (BMS) Inc.
Conflict of interest
L.Q.M. Chow, D.I. Jonker, G.K. Dy, G. Nicholas, C. Fortin, A.A. Adjei, C.P. Belani, and S.A. Laurie have no conflicts of interest to declare. D. Patricia, A. Gupta, J.-S. Park, S. Zhang, and E.I. Sbar are employees of the Bristol-Myers Squibb (BMS) Company and hold stock in BMS, the manufacturer of BMS-690514.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov Identifier: NCT00420186.
Rights and permissions
About this article
Cite this article
Chow, L.Q.M., Jonker, D.I., Dy, G.K. et al. A phase I trial to determine the safety, pharmacokinetics, and pharmacodynamics of intercalated BMS-690514 with paclitaxel/carboplatin (PC) in advanced or metastatic solid malignancies. Cancer Chemother Pharmacol 71, 1273–1285 (2013). https://doi.org/10.1007/s00280-013-2126-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2126-9