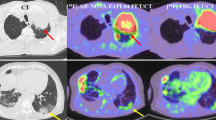
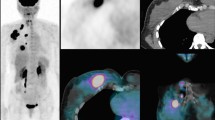
Abstract
Interventional radiological procedures for diagnosis and treatment of lung cancer have become increasingly important. Imaging-guided percutaneous biopsy has become the modality of choice for diagnosing lung cancer, and in the era of target therapies, it is an useful tool to define earlier patient-specific tumor phenotypes. In functionally inoperable patients, especially the ablative procedures are potentially curative alternatives to surgery. In addition to thermally ablative treatment, selective chemoembolization by a vascular access allows localized therapy. These treatments are considered for patients in a reduced general condition which does not allow systemic chemotherapy. The present article reviews the role of interventional oncology in the management of primary lung cancer, focusing on the state of the art for each procedure.




Similar content being viewed by others
References
Stewart BW, Wild C. World Cancer Report. Lyon: International Agency for Research on Cancer; World Health Organization; 2014.
Gangi A, Buy X. Imagerie interventionelle du cancer bronchique: du diagnostic ai traitement. Rev Mal Respir. 2007;24:6S137–45.
Priola AM, Priola SM, Cataldi A, et al. Accuracy of CT-guided transthoracic needle biopsy of lung lesions: factors affecting diagnostic yield. Radiol Med. 2007;112:1142–59.
Abi-Jaoudeh N, Duffy AG, Greten TF, et al. Personalized oncology in interventional radiology. J Vasc Interv Radiol. 2013;24:1083–92.
Fujita S, Mio T, Sonobe M, et al. Accuracy of epidermal growth factor receptor mutation analysis on the basis of small biopsy specimens in patients with nonsmall cell lung cancer. Int J Cancer. 2006;119:1751–2.
Hsieh MH, Fang YF, Chang WC, et al. Complex mutation patterns of epidermal growth factor receptor gene associated with variable responses to gefitinib treatment in patients with non-small cell lung cancer. Lung Cancer. 2006;53:311–22.
Solomon SB, Zakowski MF, Pao W, et al. Core needle lung biopsy specimens: adequacy for EGFR and KRAS mutational analysis. AJR Am J Roentgenol. 2010;194:266–9.
Yoon HJ, Lee HY, Lee KS, et al. Repeat biopsy for mutational analysis of non-small cell lung cancers resistant to previous chemotherapy: adequacy and complications. Radiology. 2012;265:939–48.
Rotolo N, Floridi C, Imperatori A, et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur Radiol. 2016;26:381–9.
O’Neill AC, McCarthy C, Ridge CA, et al. Rapid needle-out patient-rollover time after percutaneous CT-guided transthoracic biopsy of lung nodules: effect on pneumothorax rate. Radiology. 2012;262(1):314–9.
Kim JI, Park CM, Lee SM, et al. Rapid needle-out patient-rollover approach after cone beam CT-guided lung biopsy: effect on pneumothorax rate in 1,191 consecutive patients. Eur Radiol. 2015;25:1845–53.
Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology. 2002;225(2):511–8.
Pereira PL, Masala S. Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc Intervent Radiol. 2012;35(2):247–54.
Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260:633–55.
Goldberg SN, Gazelle GS, Compton CC, et al. Radiofrequency tissue ablation in the rabbit lung: efficacy and complications. Acad Radiol. 1995;2:776–84.
Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–9.
Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities—part I. J Vasc Interv Radiol. 2001;12:1021–32.
Vogl TJ, Naguib NN, Lehnert T, et al. Radiofrequency, microwave and laser ablation of pulmonary neoplasms: clinical studies and technical considerations—Review article. Eur J Radiol. 2011;77:346–57.
Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol. 2008;15:1765–74.
Kim SR, Han HJ, Park SJ, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol. 2011;81:395–9.
Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211:68–72.
Safi S, Rauch G, op den Winkel J, et al. Sublobar resection, radiofrequency ablation or radiotherapy in stage I non-small cell lung cancer. Respiration. 2015;89:550–7.
Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121:3491–8.
de Baere T, Farouil G, Deschamps F, et al. Lung cancer ablation: what is the evidence? Semin Intervent Radiol. 2013;30:151–6.
Ridge CA, Silk M, Petre EN, et al. Radiofrequency ablation of T1 lung carcinoma: comparison of outcomes for first primary, metachronous, and synchronous lung tumors. J Vasc Interv Radiol. 2014;25:989–96.
Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation using a multiple-electrode switching system for lung tumors with 2.0–5.0-cm maximum diameter: phase II clinical study. Radiology. 2015;277:895–902.
Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–75.
Palussiere J, Lagarde P, Auperin A, et al. Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: results of eight years’ experience in 87 patients from two centers. Cardiovasc Intervent Radiol. 2015;38:160–6.
Hiraki T, Gobara H, Mimura H, et al. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142:24–30.
Huang L, Han Y, Zhao J, et al. Is radiofrequency thermal ablation a safe and effective procedure in the treatment of pulmonary malignancies? Eur J Cardiothorac Surg. 2011;39:348–51.
Ambrogi MC, Fanucchi O, Cioni R, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol. 2011;6(12):2044–51.
Lanuti M, Sharma A, Willers H, et al. Radiofrequency ablation for stage I non-small cell lung cancer: management of locoregional recurrence. Ann Thorac Surg. 2012;93:921–7.
Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9:621–8.
Liu B, Liu L, Hu M, et al. vPercutaneous radiofrequency ablation for medically inoperable patients with clinical stage I non-small cell lung cancer. Thorac Cancer. 2015;6:327–33.
Carrafiello G, Mangini M, Fontana F, et al. Radiofrequency ablation for single lung tumours not suitable for surgery: seven years’ experience. Radiol Med. 2012;117:1320–32.
Carrafiello G, Mangini M, De Bernardi I, et al. Microwave ablation therapy for treating primary and secondary lung tumours: technical note. Radiol Med. 2010;115:962–74.
Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247:871–9.
Lu Q, Cao W, Huang L, Wan Y, et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: results in 69 cases. World J Surg Oncol. 2012;7(10):80.
Belfiore G, Ronza F, Belfiore MP, et al. Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol. 2013;82:177–81.
Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol. 2013;57:466–744.
Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110:758–63.
Wei Z, Ye X, Yang X, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32:464.
Wei Z, Ye X, Yang X, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–42.
Xu X, Ye X, Liu G, et al. Targeted percutaneous microwave ablation at the pulmonary lesion combined with mediastinal radiotherapy with or without concurrent chemotherapy in locally advanced non-small cell lung cancer evaluation in a randomized comparison study. Med Oncol. 2015;32:227.
Carrafiello G, Mangini M, Fontana F, et al. Microwave ablation of lung tumours: single-centre preliminary experience. Radiol Med. 2014;119:75–82.
Gage AA, Baust J. Mechanisms of tissue injury in cryo-surgery. Cryobiology. 1998;37:171–86.
Hinshaw JL, Lee FT Jr, Laeseke PF, et al. Temperature isotherms during pulmonary cryoablation and their correlation with the zone of ablation. J Vasc Intervent Radiol. 2010;21:1424–8.
Hinshaw JL, Littrup PJ, Durick N, et al. Optimizing the protocol for pulmonary cryoablation: a comparison of a dual- and triple- freeze protocol. Cardiovasc Intervent Radiol. 2010;33:1180–5.
Inoue M, Nakatsuka S, Jinzaki M. Cryoablation of early-stage primary lung cancer. Biomed Res Int. 2014;2014:52–1691.
Yamauchi Y, Izumi Y, Hashimoto K, et al. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS ONE. 2012;7:e33223.
Zhang X, Tian J, Zhao L, et al. CT-guided conformal cryoablation for peripheral NSCLC: initial experience. Eur J Radiol. 2012;81:3354–62.
Pusceddu C, Sotgia B, Fele RM et al (2013) CT-guided thin needles percutaneous cryoablation (PCA) in patients with primary and secondary lung tumors: a preliminary experience. Eur J Radiol 82:246–e253.
Yashiro H, Nakatsuka S, Inoue M, et al. Factors affecting local progression after percutaneous cryoablation of lung tumors. J Vasc Intervent Radiol. 2013;24:813–21.
Golder WA. Chemoembolization of the lungs: basic anatomical elements, experimental results, clinical experience. Onkologe. 2008;14:934–9.
Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92.
Walker CM, Rosado-de-Christenson ML, Martínez-Jiménez S, et al. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics. 2015;35:32–49.
Vogl TJ, Shafinaderi M, Zangos S, et al. Regional chemotherapy of the lung: transpulmonary chemoembolization in malignant lung tumors. Semin Intervent Radiol. 2013;30:176–84.
Franke UF, Wittwer T, Lessel M, et al. Evaluation of isolated lung perfusion as neoadjuvant therapy of lung metastases using a novel in vivo pig model: i. Influence of perfusion pressure and hyperthermia on functional and morphological lung integrity. Eur J Cardiothorac Surg. 2004;26:792–9.
Schneider P, Kampfer S, Loddenkemper C, et al. Chemoembolization of the lung improves tumor control in a rat model. Clin Cancer Res. 2002;8:2463–8.
Lindemayr S, Lehnert T, Korkusuz H, et al. Transpulmonary chemoembolization: a novel approach for the treatment of unresectable lung tumors. Tech Vasc Intervent Rad. 2007;10:114–9.
Baylatry MT, Pelage JP, Wassef M, et al. Pulmonary artery chemoembolization in a sheep model: evaluation of performance and safety of irinotecan eluting beads (DEB-IRI). J Biomed Mater Res B Appl Biomater. 2011;98:351–9.
Park HS, Kim YI, Kim HY, et al. Bronchial artery and systemic artery embolization in the management of primary lung cancer patients with hemoptysis. Cardiovasc Intervent Radiol. 2007;30:638–43.
Garcia-Olivé I, Sanz-Santos J, Centeno C, et al. Results of bronchial artery embolization for the treatment of hemoptysis caused by neoplasm. J Vasc Interv Radiol. 2014;25:221–8.
Prévost JB, Nuyttens JJ, Hoogeman MS, et al. Endovascular coils as lung tumour markers in real-time tumour tracking stereotactic radiotherapy: preliminary results. Eur Radiol. 2008;18:1569–76.
Karaman K, Dokdok AM, Karadeniz O, et al. Intravascular placement of metallic coils as lung tumor markers for cyberknife stereotactic radiation therapy. Korean J Radiol. 2015;16:626–31.
Mallick R, Demmy T. Regional lung chemotherapy techniques. Innovations (Phila). 2011;6:1–9.
Nakanishi M, Demura Y, Umeda Y, et al. Multi-arterial infusion chemotherapy for non-small cell lung carcinoma–significance of detecting feeding arteries and tumor staining. Lung Cancer. 2008;61:227–34.
Demmy TL, Tomaszewski G, Dy GK, et al. Thoracoscopic organ suffusion for regional lung chemotherapy (preliminary results). Ann Thorac Surg. 2009;88:385–90.
Ferris EJ. Pulmonary hemorrhage. Vascular evaluation and interventional therapy. Chest. 1981;80:710–4.
Chun JY, Morgan R, Belli AM, et al. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010;33:240–50.
Hahn S, Kim YJ, Kwon W, et al. Comparison of the effectiveness of embolic agents for bronchial artery embolization: gelfoam versus polyvinyl alcohol. Korean J Radiol. 2010;11:542–6.
Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22:1395–409.
Baltacioglu F, Cimsit NC, Bostanci K, et al. Transarterial microcatheter glue embolization of the bronchial artery for life-threatening hemoptysis: technical and clinical results. Eur J Radiol. 2010;73:380–4.
Razavi MK, Murphy K. Embolization of bronchial arteries with N-butyl cyanoacrylate for management of massive hemoptysis: a technical review. Tech Vasc Interv Radiol. 2007;10:276–82.
Woo S, Yoon CJ, Chung JW, et al. Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology. 2013;269:594–602.
Andersen PE. Imaging and interventional radiological treatment of hemoptysis. Acta Radiol. 2006;47:780–92.
Ittrich H, Klose H, Adam G. Radiologic management of haemoptysis: diagnostic and interventional bronchial arterial embolisation. Fortschr Röntgenstr. 2015;187:248–59.
Nakanishi M, Umeda Y, Demura Y, et al. Effective use of multi-arterial infusion chemotherapy for advanced non-small cell lung cancer patients: four clinical specified cases. Lung Cancer. 2007;55:241–7.
Nakanishi M, Yoshida Y, Natazuka T. Prospective study of transarterial infusion of docetaxel and cisplatin to treat non-small-cell lung cancer in patients contraindicated for standard chemotherapy. Lung Cancer. 2012;77:353–8.
Yuan Z, Li WT, Ye XD, et al. Intra-arterial infusion chemotherapy for advanced non-small-cell lung cancer: preliminary experience on the safety, efficacy, and clinical outcomes. J Vasc Interv Radiol. 2013;24(1521–8):e4.
Yim CD, Sane SS, Bjarnason H. Superior vena cava stenting. Radiol Clin North Am. 2000;38:409–24.
Fagedet D, Thony F, Timsit JF, et al. Endovascular treatment of malignant superior vena cava syndrome: results and predictive factors of clinical efficacy. Cardiovasc Intervent Radiol. 2013;36:140–9.
Lanciego C, Pangua C, Chacón JI, et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2009;193:549–58.
Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol. 2006;29:319–22.
Bierdrager E, Lampmann LEH, Lohle PNM, et al. Endovascular stenting in neoplastic superior vena cava syndrome prior to chemotherapy or radiotherapy. Neth J Med. 2005;63:20–3.
Nagata T, Makutani S, Uchida H, et al. Follow-up results of 71 patients undergoing metallic stent placement for the treatment of a malignant obstruction of the superior vena cava. Cardiovasc Intervent Radiol. 2007;30:959–67.
Gwon DI, Ko GY, Kim JH, et al. Malignant superior vena cava syndrome: a comparative cohort study of treatment with covered stents versus uncovered stents. Radiology. 2013;266:979–87.
Maleux G, Gillardin P, Fieuws S, et al. Large-bore nitinol stents for malignant superior vena cava syndrome: factors influencing outcome. AJR Am J Roentgenol. 2013;201:667–74.
Sobrinho G, Aguiar P. Stent placement for the treatment of malignant superior vena cava syndrome—a single-center series of 56 patients. Arch Bronconeumol. 2014;50:135–40.
Andersen PE, Duvnjak S. Palliative treatment of superior vena cava syndrome with nitinol stents. Int J Angiol. 2014;23:255–62.
Cho Y, Gwon DI, Ko GY, et al. Covered stent placement for the treatment of malignant superior vena cava syndrome: is unilateral covered stenting safe and effective? Korean J Radiol. 2014;15:87–94.
Andersen PE, Midtgaard A, Brenöe AS, et al. A new nitinol stent for use in superior vena cava syndrome. Initial clinical experience. J Cardiovasc Surg. 2015;56:877–81.
Mokry T, Bellemann N, Sommer CM, et al. Retrospective study in 23 patients of the self-expanding sinus-XL stent for treatment of malignant superior vena cava obstruction caused by non-small cell lung cancer. J Vasc Interv Radiol. 2015;26:357–65.
Gulati A, Shah R, Puttanniah V, et al. A retrospective review and treatment paradigm of interventional therapies for patients suffering from intractable thoracic chest wall pain in the oncologic population. Pain Med. 2015;16:802–10.
Wong FC, Lee TW, Yuen KK, et al. Intercostal nerve blockade for cancer pain: effectiveness and selection of patients. Hong Kong Med J. 2007;13:266–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ejona Duka, Anna Maria Ierardi, Chiara Floridi, Alberto Terrana, Federico Fontana, Gianpaolo Carrafiello have no conflict of interest.
Ethical approval
“All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Duka, E., Ierardi, A.M., Floridi, C. et al. The Role of Interventional Oncology in the Management of Lung Cancer. Cardiovasc Intervent Radiol 40, 153–165 (2017). https://doi.org/10.1007/s00270-016-1495-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-016-1495-y