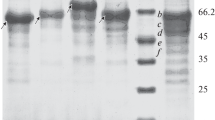
Abstract
Two high-level heterologous expression systems for amylosucrase genes have been constructed. One depends on sigma-70 bacterial RNA polymerase, the other on phage T7 RNA polymerase. Translational fusions were formed between slightly truncated versions of the gene from Neisseria polysaccharea and sequences of expression vectors pQE-81L or pET33b(+), respectively. These constructs were introduced into different Escherichia coli strains. The resulting recombinants yielded up to 170 mg of dissolved enzyme per litre of culture at a moderate cell density of five OD600. To our knowledge, this is the highest yield per cell described so far for amylosucrases. The recombinant enzymes could rapidly be purified through the use of histidine tags in the N-terminally attached sequences. These segments did not alter catalytic properties and therefore need not be removed for most applications. Investigations with glucose and malto-oligosaccharides of different lengths identified rate-limiting steps in the elongation (acceptor reaction) and truncation (donor reaction) of these substrates. The elongation of maltotriose and its reversal, the truncation of maltotetraose, were found to be particularly slow reactions. Potential reasons are discussed, based on the crystal structure of the enzyme. It is furthermore shown that amylosucrase is able to synthesise mixed disaccharides. All of the glucose epimers mannose, allose, and galactose served as acceptors, yielding between one and three main products. We also demonstrate that, as an alternative to the use of purified amylosucrase, cells of the constructed recombinant strains can be used to carry out glucosylations of acceptors.





Similar content being viewed by others
References
Albenne C, Skov LK, Mirza O, Gajhede M, Potocki-Véronèse G, Monsan P, Remaud-Siméon M (2002) Maltooligosaccharide disproportionation reaction: an intrinsic property of amylosucrase from Neisseria polysaccharea. FEBS Lett 527:67–70
Albenne C, Skov LK, Mirza O, Gajhede M, Feller G, D’Amico S, André G, Potocki-Véronèse G, van der Veen BA, Monsan P, Remaud-Siméon M (2004) Molecular basis of the amylose-like polymer formation catalyzed by Neisseria polysaccharea amylosucrase. J Biol Chem 279:726–734
Bartels F, Backhaus S, Moore ERB, Timmis KN, Hofer B (1999) Occurrence and expression of glutathione S-transferase-encoding bphK genes in Burkholderia sp. strain LB400 and other biphenyl-utilizing bacteria. Microbiology (UK) 145:2821–2834
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Büttcher V, Welsh T, Willmitzer L, Kossmann J (1997) Cloning and characterization of the gene for amylosucrase from Neisseria polysaccharea: production of a linear α-1, 4-glucan. J Bacteriol 179:3324–3330
Champion E, André I, Moulis C, Boutet J, Descroix K, Morel S, Monsan P, Mulard LA, Remaud-Siméon M (2009) Design of α-transglucosidases of controlled specificity for programmed chemoenzymatic synthesis of antigenic oligosaccharides. J Am Chem Soc 131:7379–7389
DeLano WL (2007) Available at http://www.pymol.org
Grant SGN, Jessee J, Bloom FR, Hanahan D (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci USA 87:4645–4649
Ha S-J, Seo D-H, Jung J-H, Cha J, Kim T-J, Kim YW, Park C-S (2009) Molecular cloning and functional expression of a new amylosucrase from Alteromonas macleodii. Biosci Biotechnol Biochem 73:1505–1512
Hehre EJ, Hamilton DM, Carlson AS (1949) Synthesis of a polysaccharide of the starch-glycogen class from sucrose by a cell-free, bacterial enzyme system (amylosucrase). J Biol Chem 177:267–279
Henrissat B (1991) A classification of glycosyl hydrolases based on amino-acid sequence similarities. Biochem J 280:309–316
Henrissat B, Davies GJ (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Op Struct Biol 7:637–644
Jensen MH, Mirza O, Albenne C, Remaud-Simeon M, Monsan P, Gajhede M, Skov LK (2004) Crystal structure of the covalent intermediate of amylosucrase from Neisseria polysaccharea. Biochemistry 43:3104–3110
MacKenzie CR, Perry MB, McDonald IJ, Johnson KG (1978) Structure of the d-glucans produced by Neisseria perflava. Can J Microbiol 24:1419–1422
Mayer RM (1987) Dextransucrase: a glucosyltransferase from Streptococcus sanguis. Methods Enzymol 138:649–661
Mirza O, Skov LK, Remaud-Siméon M, Potocki de Montalk G, Albenne C, Monsan P, Gajhede M (2001) Crystal structures of amylosucrase from Neisseria polysaccharea in complex with d-glucose and the active site mutant Glu328Gln in complex with the natural substrate sucrose. Biochemistry 40:9032–9039
Mukerjea R, Kim D, Robyt JF (1996) Simplified and improved methylation analysis of saccharides, using a modified procedure and thin-layer chromatography. Carbohydr Res 292:11–20
Okada G, Hehre EJ (1974) New studies on amylosucrase, a bacterial a-d-glucosylase that directly converts sucrose to a glycogen-like a-glucan. J Biol Chem 249:126–135
Pizzut-Serin S, Potocki-Véronèse G, van der Veen BA, Albenne C, Monsan P, Remaud-Siméon M (2005) Characterisation of a novel amylosucrase from Deinococcus radiodurans. FEBS Lett 579:1405–1410
Potocki de Montalk G, Remaud-Siméon M, Willemot RM, Planchot V, Monsan P (1999) Sequence analysis of the gene encoding amylosucrase from Neisseria polysaccharea and characterization of the recombinant enzyme. J Bacteriol 181:375–381
Potocki de Montalk G, Remaud-Siméon M, Willemot RM, Monsan P (2000a) Characterisation of the activator effect of glycogen on amylosucrase from Neisseria polysaccharea. FEMS Microbiol Lett 186:103–108
Potocki de Montalk G, Remaud-Siméon M, Willemot RM, Sarçabal P, Planchot V, Monsan P (2000b) Amylosucrase from Neisseria polysaccharea: novel catalytic properties. FEBS Lett 471:219–223
Riou JY, Guibourdenche M, Popoff MY (1983) A new taxon in the genus Neisseria. Ann Inst Pasteur Microbiol 134:257–267
Robyt JF, Mukerjea R (1994) Separation and quantitative determination of nanogram quantities of maltodextrins and isomaltodextrins by thin-layer chromatography. Carbohydr Res 251:187–202
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor, Cold Spring Harbor Laboratory
Schneider J, Fricke C, Overwin H, Hofmann B, Hofer B (2009) Generation of amylosucrase variants that terminate catalysis of acceptor elongation at the di- or trisaccharide stage. Appl Environ Microbiol 75:7453–7460
Skov LK, Mirza O, Henriksen A, Potocki de Montalk G, Remaud-Siméon M, Sarçabal P, Willemot RM, Monsan P, Gajhede M (2001) Amylosucrase, a glucan-synthesizing enzyme from the alpha-amylase family. J Biol Chem 276:25273–25278
Skov LK, Mirza O, Sprogoe D, Dar I, Remaud-Siméon M, Albenne C, Monsan P, Gajhede M (2002) Oligosaccharide and sucrose complexes of amylosucrase. Structural implications for the polymerase activity. J Biol Chem 277:47741–47747
Swistowska AM, Gronert S, Wittrock S, Collisi W, Hecht H-J, Hofer B (2007) Identification of structural determinants for substrate binding and turnover by glucosyltransferase R supports the permutation hypothesis. FEBS Lett 581:4036–4042
Swistowska AM, Wittrock S, Collisi W, Hofer B (2008) Heterologous hyper-expression of a glucansucrase type glycosyltransferase gene. Appl Microbiol Biotechnol 79:255–261
Tao BY, Reilly PJ, Robyt JF (1988) Neisseria perflava amylosucrase: characterization of its product polysaccharide and a study of its inhibition by sucrose derivatives. Carbohydr Res 181:163–173
Zhu P, Tsang RSW, Tsai C-M (2003) Nonencapsulated Neisseria meningitidis strain produces amylopectin from sucrose: altering the concept for differentiation between N. meningitidis and N. polysaccharea. J Clin Microbiol 41:273–278
Acknowledgements
Financial support of parts of this work by the Deutsche Forschungsgemeinschaft through SFB 578, project A3, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneider, J., Fricke, C., Overwin, H. et al. High level expression of a recombinant amylosucrase gene and selected properties of the enzyme. Appl Microbiol Biotechnol 89, 1821–1829 (2011). https://doi.org/10.1007/s00253-010-3000-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-3000-x