Abstract
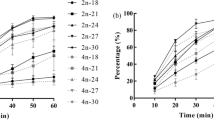
Early development was examined, under various salinities, for two sympatric nereidid polychaetes, Hediste japonica and H. diadroma, which participated in a simultaneous reproductive swarming in an estuary of the Omuta-gawa River in Ariake Sea, Japan. The eggs of both species were isotonic to the medium of 27.5–30 psu salinity. The egg diameter in the isotonic salinity was 180–205 μm in H. japonica, and 130–160 μm in H. diadroma. Successful development of most embryos was observed in a salinity range of 22.5–30 psu in both species, while successful fertilization occurred in wider ranges of salinity, i.e., 10–34 psu in H. japonica and 10 to 30 psu in H. diadroma. In both species, free-swimming larval life started from the indistinct hatching of trochophores out of a jelly layer capsule. The lecithotrophic development appeared to run to the 4-setiger nectochaetes in H. japonica, while to 3-setiger nectochaetes in H. diadroma, resulting in a shorter pelagic larval life in H. japonica. In a comparison of larval morphology among Hediste species, we found a definite negative correlation between the prostomium width, which represents the larval size and depends on egg size, and relative length of chaetae to the prostomium width: the relative length of chaetae was the longest in H. diadroma (with the smallest egg size and long pelagic life), intermediate in H. japonica (intermediate egg size, short pelagic life), and the shortest in H. atoka (largest egg size, no true pelagic life). We also examined the possibility of hybridization between H. japonica and H. diadroma through cross-insemination experiments. The gametes of the two species were reciprocally compatible, and viable hybrid offspring were produced by the laboratory crosses. The hybrid larvae expressed intermediate phenotypes, but with a greater maternal influence in characteristics such as the relative length of chaetae and the lecithotrophic larval duration.











Similar content being viewed by others
References
Bartels-Hardege HD, Hardege JD, Zeeck E, Muller C, Wu BL, Zhu MY (1996) Sex pheromones in marine polychaetes V: a biologically active volatile compound from the coelomic fluid of female Nereis (Neanthes) japonica (Annelida Polychaeta). J Exp Mar Biol Ecol 201:275–284
Boilly-Marer Y (1974) Etude experimentale du comportement nuptial de Platynereis dumerilii (Annelida: Polychaeta): chemoreception, emission des produits genitaux. Mar Biol 24:167–179
Byrne M, Anderson MJ (1994) Hybridization of sympatric Patiriella species (Echinodermata: Asteroidea) in New South Wales. Evolution 48:564–576
Clark RB (1961) The origin and formation of the heteronereis. Biol Rev 36:199–236
Gardner JPA (1994) The structure and dynamics of naturally occurring hybrid Mytilus edulis Linnaeus, 1758 and Mytilus galloprovincialis Lamarck, 1819 (Bivalvia: Mollusca) populations: review and interpretation. Arch Hydrobiol 99(Suppl):37–71
Hardege JD (1999) Nereidid polychaetes as model organisms for marine chemical ecology. Hydrobiologia 402:145–161
Hardege JD, Bartels-Hardege H (1995) Spawning behaviour and development of Perinereis nuntia var. brevicirrus (Annelida: Polychaeta). Invertebr Biol 114:39–45
Hodgson G (1988) Potential gamete wastage in synchronously spawning corals due to hybrid inviability. Proc 6th Int Coral Reef Symp 2:707–714
Inamori Y, Kurihara Y (1979) Analysis of the environmental factors affecting the life of the brackish polychaete, Neanthes japonica (Izuka). III. The effects of the environmental factors on fertilization, cleavage and postlarval development. Bull Mar Biol Stn Asamushi 16:113–121
Izuka A (1908) On the breeding habit and development of Nereis japonica n. sp. Annot Zool Jpn 6:295–305
Kagawa Y (1955) Note on the optimum salinities, studied in the adult and larva of the brackish-water polychaete worm, Nereis japonica. J Gakugei Coll, Tokushima Univ, Nat Sci 6:11–16 (in Japanese with English summary)
Kwast KE, Foltz DW, Stickle WB (1990) Population genetics and systematics of the Leptasterias hexactis (Echinodermata: Asteroidea) species complex. Mar Biol 105:477–489
Levin LA (1984) Multiple patterns of development in Streblospio benedicti Webster (Spionidae) from three coasts of North America. Biol Bull 166:494–508
Lessios HA, Cunningham CW (1990) Gametic incompatibility between species of the sea urchin Echinometra on the two sides of the Isthmus of Panama. Evolution 44:933–941
Lillie FR, Just EF (1913) Breeding habits of the heteronereis form of Nereis limbata at Whitstable Massachusetts. Biol Bull 24:147–160
Marsden JR (1992) Reproductive isolation in two forms of the serpulid polychaete Spirobranchus polycerus in Berbados. Bull Mar Sci 51:14–18
Miller K, Babcock RC (1997) Conflicting morphological and reproductive species boundaries in the coral genus Platygyra. Biol Bull 192:98–110
Palumbi SR, Metz EC (1991) Strong reproductive isolation between closely related tropical sea urchins (genus Echinometra). Mol Biol Evol 8:227–239
Pearse JS, McClary DJ, Sewell MA, Austin WC, Perez-Ruzafa A, Byrne M (1988) Simultaneous spawning of six species of echinoderms in Barkley Sound, British Columbia. Invertebr Reprod Dev 14:279–288
Pernet B (1999) Gamete interactions and genetic differentiation among three sympatric polychaetes. Evolution 53:435–446
Pennington JT, Chia FS (1984) Morphological and behavioral defenses of trochophore larvae of Sabellaria cementarium (Polychaeta) against four planktonic predators. Biol Bull 167:168–175
Sato M (1999) Divergence of reproductive and developmental characteristics in Hediste (Polychaeta: Nereididae). Hydrobiologia 402:129–143
Sato M, Nakashima A (2003) A review of Asian Hediste species complex (Nereididae, Polychaeta) with descriptions of two new species and a redescription of Hediste japonica (Izuka, 1908). Zool J Linn Soc 137:403–445
Sato M, Tsuchiya M (1987) Reproductive behavior and salinity favorable for early development in two types of the brackish-water polychaete Neanthes japonica (Izuka). Benthos Res (Japan) 31:29–42
Sato M, Tsuchiya M (1991) Two patterns of early development in nereidid polychaetes keying out of Neanthes japonica (Izuka). Ophelia 5(Suppl):371–382
Schopf TJM, Murphy LS (1973) Protein polymorphism of the hybridizing seastars Asterias forbesi and Asrerias vulgaris and implications for their evolution. Biol Bull 145:589–597
Strathmann RR (1981) On barriers to hybridization between between Strongylocentrotus droebachiensis (O.F. Müller) and S. Pallidus (G.O. Sars). J Exp Mar Bio Ecol 55:39–47
Tosuji H, Miyamoto J, Hayata Y, Sato M (2004) Karyotyping of female and male Hediste japonica (Polychaeta, Annelida) in comparison with those of two closely related species, H. diadroma and H. atoka. Zool Sci 21:147–152
Wallace CC, Willis BL (1994) Systematics of the coral genus Acropora: Implications of new biological findings for species concepts. Annu Rev Ecol Syst 25:237–262
Zeeck E, Hardege J, Bartels-Hardege H (1990) Sex pheromones and reproductive isolation in two nereid species, Nereis succinea and Platynereis dumerilii. Mar Ecol Prog Ser 67:183–188
Acknowledgments
We would like to thank Hidetoshi Nakashima of the Omuta Zoo for assistance in collecting animals, and Simon P. Varnam (Shizuoka, Japan) for English advice. This research was supported by a grant from the Research Institute of Marine Invertebrates (RIMI), Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Ikeda, Hakodate
Rights and permissions
About this article
Cite this article
Tosuji, H., Sato, M. Salinity favorable for early development and gamete compatibility in two sympatric estuarine species of the genus Hediste (Polychaeta: Nereididae) in the Ariake Sea, Japan. Marine Biology 148, 529–539 (2006). https://doi.org/10.1007/s00227-005-0079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0079-1