Abstract
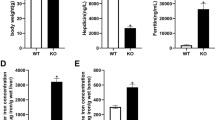
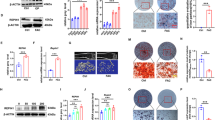
It has been found that iron disorder may lead to osteoporosis. However, the mechanism has been little explored. In the present study, we try to investigate the effects of iron disorder on bone metabolism using Irp2 knockout (Irp2−/−) mice. Female Irp2−/− mice were used in this study. Bone mineral density (BMD) was measured by Micro-CT. Serum markers for bone turnover were measured by enzyme-linked immunosorbent assay. Content of iron was measured in bone and liver tissue, and Vitamin D 25-hydroxylase (CYP2R1) content was measured in liver tissue. Relative gene expression involved in iron export and uptake, and some genes involved in activities of osteoblast and osteoclast were all measured by real-time PCR and western blot. Compared to wild-type mice, Irp2−/− mice exhibited reduced BMD, bone iron deficiency, and hepatic iron overload. Serum levels of 25(OH)D3 and markers for bone formation such as bone alkaline phosphatase (Balp), bone-gla-protein (BGP), and type I collagen alpha1 chain (Col I α1) were decreased, while markers for bone resorption including cathepsin K (Ctsk) and tartrate-resistant acid phosphatase (Trap) were all significantly increased. Hepatic CYP2R1 level was decreased in Irp2−/− mice compared with wild-type control mice. Compared to wild-type C57BL6 control mice, the expression of genes involved in osteoblast activity such as Balp, BGP, and Col I α1 were all significantly decreased in bone tissue, while genes for osteoclast activity such as Ctsk and Trap were all markedly increased in Irp2−/− mice at mRNA level. Genes involved in iron storage, uptake, and exporting were also measured in bone tissue. Posttranscriptionally decreased ferritin (FTL), ferroportin 1 (FPN1), and increased transferrin receptor 1 (TfR1) gene expressions have been unexpectedly found in bone tissue of Irp2−/− mice. Irp2−/− mice exhibit reduced bone iron content and osteoporosis. Decreased circulating 25(OH)D3 levels promoted activity of osteoclast, while impaired activity of osteoblast may contribute to pathogenesis of osteoporosis. And, reduced bone iron content may not be totally caused by TfR1-dependent pathways.




Similar content being viewed by others
References
Rossi F, Perrotta S, Bellini G et al (2014) Iron overload causes osteoporosis in thalassemia major patients through interaction with transient receptor potential vanilloid type 1 (TRPV1) channels. Haematologica 99(12):1876–1884
Guggenbuhl P, Deugnier Y, Boisdet JF et al (2005) Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 16:1809–1814
Valenti L, Varenna M, Fracanzani AL (2009) et, al. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int 20:549–555
Sinigaglia L, Fargion S, Fracanzani AL et al (1997) Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol 24(9):1809–1813
Medeiros DM, Plattner A, Jennings D et al (2002) Bone morphology, strength and density are compromised in iron-deficient rats and exacerbated by calcium restriction. J Nutr 132(10):3135–3141
Medeiros DM, Stoecker B, Plattener A et al (2004) Iron deficiency negatively affects vertebrae and femurs of rats independently of energy intake and body weight. J Nutr 134(11):3061–3067
Li GF, Pan YZ, Sirois P et al (2012) Iron homeostasis in osteoporosis and its clinical implications. Osteoporos Int 23:2403–2408
Yamasaki K, Hagiwara H (2009) Excess iron inhibits osteoblast metabolism. Toxicol Lett 191:211–215
He YF, Ma Y, Gao C et al (2013) Iron overload inhibits osteoblast biological activity through oxidative stress. Biol Trace Elem Res 152(2):292–296
Hentze MW, Muckenthaler MU, Galy B et al (2010) Two to tango:regulation of Mammalian iron metabolism. Cell 9(1):24–38 142(
Anderson CP, Shen M, Eisenstein RS et al (2012) Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta 1823(9):1468–1483
Sanchez M, Galy B, Schwanhaeusser B et al (2011) Iron regulatory protein-1 and -2: transcriptome-wide definition of binding mRNAs and shaping of the cellular proteome by iron regulatory proteins. Blood 118(22):e168–e179
Vashisht AA, Zumbrennen KB, Huang X et al (2009) Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 326(5953):718–721
Salahudeen AA, Thompson JW, Ruiz JC et al (2009) An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science 326(5953):722–726
Moroishi T, Nishiyama M, Takeda Y et al (2011) The FBXL5-IRP2 axis is integral to control of iron metabolism in vivo. Cell Metab 14(3):339–351
Ishii KA, Fumoto T, Iwai K et al (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15(3):259–266
Yang Q, Jian J, Abramson SB et al (2011) Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res 26:1188–1196
Chang YZ, Qian ZM, Wang K et al (2005) Effects of development and iron status on ceruloplasmin expression in rat brain. J Cell Physiol 204(2):623–631
Machado I, Bergmann G, Pistón M (2016) A simple and fast ultrasound-assisted extraction procedure for Fe and Zn determination in milk-based infant formulas using flame atomic absorption spectrometry (FAAS). Food Chem 194:373–376
Shi ZH, Nie G, Duan XL, Rouault T, Wu WS, Ning B, Zhang N, Chang YZ, Zhao BL (2010) Neuroprotective mechanism of mitochondrial ferritin on 6-hydroxydopamine-induceddopaminergic cell damage: implication for neuroprotection in Parkinson’s disease. Antioxid Redox Signal 2010;13(6):783–796
Winter WE, Bazydlo LA, Harris NS (2014) The molecular biology of human iron metabolism. Lab Med 45(2):92–102
Li J, Hou Y, Zhang S et al (2013) Excess iron undermined bone load-bearing capacity through tumor necrosis factor-alpha-dependent osteoclastic activation in mice. Biomed Rep 1:85–88
D’Amelio P, Cristofaro MA, Tamone C et al (2008) Role of iron metabolism and oxidative damage in postmenopausal bone loss. Bone 43:1010–1015
Meyron-Holtz EG, Ghosh MC, Rouault TA (2004) Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo. Science 306:2087–2090
Meyron-Holtz EG, Ghosh MC, Iwai K et al (2004) Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. EMBO J 23:386–395
Kim HY, Klausner RD, Rouault TA (1995) Translational repressor activity is equivalent and is quantitatively predicted by in vitro RNA binding for two iron-responsive element binding proteins, IRP1 and IRP2. J Biol Chem 270:4983–4986
Ghosh MC, Zhang DL, Jeong SY et al (2013) Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2α. Cell Metab 17(2):271–281
Cooperman SS, Meyron-Holtz EG, Olivierre-Wilson H et al (2005) Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood 106(3):1084–1091
Zhou J, Ye S, Fujiwara T et al (2013) Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J Biol Chem 288(42):30064–30074
Guggenbuhl P, Fergelot P, Doyard M et al (2011) Bone status in a mouse model of genetic hemochromatosis. Osteoporos Int 22(8):2313–2319
Doyard M, Chappard D, Leroyer P et al (2016) Decreased bone formation explains osteoporosis in a genetic mouse model of hemochromatosiss. PLoS ONE 11(2):e0148292
Mediero A, Cronstein BN (2013) Adenosine and bone metabolism. Trends Endocrinol Metab 24(6):290–300
Uemura H, Yasui T, Kiyokawa M et al (2002) Serum osteoprotegerin/osteoclastogenesis-inhibitory factor during pregnancy and lactation and the relationship with calcium-regulating hormones and bone turnover markers. J Endocrinol 174(2):353–359
Price PA, Parthemore JG, Deftos LJ (1980) New biochemical marker for bone metabolism. Measurement by radioimmunoassay of bone GLA protein in the plasma of normal subjects and patients with bonedisease. J Clin Invest 66(5):878–883
Zoch ML, Clemens TL, Riddle RC (2016) New insights into the biology of osteocalcin. Bone 82:42–49
Song YE, Tan H, Liu KJ et al (2011) Effect of fluoride exposure on bone metabolism indicators ALP, BALP, and BGP. Environ Health Prev Med 16(3):158–163
Kemper O, Herten M, Fischer J et al (2014) Prostacyclin suppresses twist expression in the presence of indomethacin in bone marrow-derived mesenchymal stromal cells. Med Sci Monit 20:2219–2227
Inui T, Ishibashi O, Inaoka T et al (1997) Cathepsin K antisense oligodeoxynucleotide inhibits osteoclastic bone resorption. J Biol Chem 272(13):8109–8112
Galy B, Ferring D, Minana B et al (2005) Altered body iron distribution and microcytosis in mice deficient in iron regulatory protein 2 (IRP2). Blood 106(7):2580–2589
Arosio P, Elia L, Poli M (2017) Ferritin, cellular iron storage and regulation. IUBMB Life 69(6):414–422
Ganz T (2005) Cellular iron: ferroportin is the only way out. Cell Metab 1(3):155–157
Rochette L, Gudjoncik A, Guenancia C et al (2015) The iron-regulatory hormone hepcidin: a possible therapeutic target? Pharmacol Ther 146:35–52
Wang G, Shao A, Hu W et al (2015) Changes of ferrous iron and its transporters after intracerebral hemorrhage in rats. Int J Clin Exp Pathol 8(9):10671–10679
Rice AE, Mendez MJ, Hokanson CA et al (2009) Investigation of the biophysical and cell biological properties of ferroportin, a multipass integral membrane protein iron exporter. J Mol Biol 386(3):717–732
Zumbrennen-Bullough KB, Becker L, Garrett L et al (2014) Abnormal brain iron metabolism in Irp2 deficient mice is associated with mild neurological and behavioral impairments. PLoS ONE 9(6):e98072
Acknowledgements
We acknowledge the Beijing Synchrotron Radiation Facility for the beam time. This work was supported by the National Natural Science Foundation of China (Grant Number 31471035).
Author information
Authors and Affiliations
Contributions
YZ and YY performed most of the experiments; YL, HC, KL, and XZ performed experiments and data analysis; YY wrote the manuscript; YZ, YL, and YC interpreted data and critically revised the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Yaru Zhou, Yu Yang, Yan Liu, Hengrui Chang, Kuanzhi Liu, Xiaojuan Zhang, and Yanzhong Chang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of Hebei Science and Technical Bureau in China.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, Y., Yang, Y., Liu, Y. et al. Irp2 Knockout Causes Osteoporosis by Inhibition of Bone Remodeling. Calcif Tissue Int 104, 70–78 (2019). https://doi.org/10.1007/s00223-018-0469-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0469-2