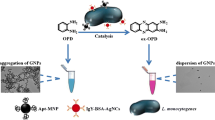
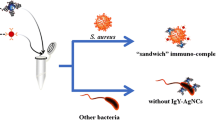
Abstract
Listeria monocytogenes is a recognized foodborne pathogen that causes listeriosis in susceptible consumers. Currently, the detection systems for Listeria in food detect live and dead bacteria, being the viable microorganisms most relevant for their ability to cause sickness in the population at risk. For this reason, a new nanohybrid compound was developed for the optical detection of Listeria that was based on polyamidoamine dendrimers functionalized with an auxotrophic cofactor (lipoic acid), together with the coupling of fluorescent semiconductor crystals (quantum dots). The nanohybrid sensor has a detection limit for viable L. monocytogenes of 5.19 × 103 colony-forming units per milliliter under epifluorescence microscopy. It was specific when used among other pathogens commonly found in food.










Similar content being viewed by others
References
Välima A, Tilsala-Timisjärvi A, Virtanen E. Rapid detection and identification methods for Listeria monocytogenes in the food chain – a review. Food Control. 2015;55:103–14. doi:10.1016/j.foodcont.2015.02.037.
Walker SJ, Archer P, Banks JG. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990;68(2):157–62.
Sauders BD, Overdevest J, Fortes E, Windham K, Schukken Y, Lembo A, et al. Diversity of Listeria species in urban and natural environments. Appl Environ Microbiol. 2012;78(12):4420–33. doi:10.1128/aem.00282-12.
Silk BJ, Date KA, Jackson KA, Pouillot R, Holt KG, Graves LM, et al. Invasive listeriosis in the Foodborne Diseases Active Surveillance Network (FoodNet), 2004-2009: further targeted prevention needed for higher-risk groups. Clin Infect Dis. 2012;54 Suppl 5:S396–404. doi:10.1093/cid/cis268.
Schlech 3rd WF, Lavigne PM, Bortolussi RA, Allen AC, Haldane EV, Wort AJ, et al. Epidemic listeriosis—evidence for transmission by food. N Engl J Med. 1983;308(4):203–6. doi:10.1056/nejm198301273080407.
Crowe SJ, Mahon BE, Vieira AR, Gould LH. Vital signs: multistate foodborne outbreaks - United States, 2010-2014. MMWR Morb Mortal Wkly Rep. 2015;64(43):1221–5. doi:10.15585/mmwr.mm6443a4.
Dwivedi HP, Jaykus LA. Detection of pathogens in foods: the current state-of-the-art and future directions. Crit Rev Microbiol. 2011;37(1):40–63. doi:10.3109/1040841x.2010.506430.
Hitchins A, Jinneman K. Laboratory methods - BAM: detection and enumeration of Listeria monocytogenes. US Food and Drug Administration, Silver Spring. http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071400.htm (2016). Accessed 4 Apr 16.
Soejima T, Iida K, Qin T, Taniai H, Seki M, Yoshida S. Method to detect only live bacteria during PCR amplification. J Clin Microbiol. 2008;46(7):2305–13. doi:10.1128/jcm.02171-07.
Koyun A, Ahlatcıoğlu E, İpek YK. Biosensors and their principles. In: Kara S, editor. A roadmap of biomedical engineers and milestones. Rijeka: In Tech; 2012. 10.5772/48824
Chekina C, Horák D, Jendelová P, Trchová M, Beneš M, Hrubý M, et al. Fluorescent magnetic nanoparticles for biomedical applications. J Mater Chem. 2011;21:7630–9. doi:10.1039/C1JM10621J.
Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22(8):969–76. doi:10.1038/nbt994.
Wang L, Zhao W, Tan W. Bioconjugated silica nanoparticles: development and applications. Nano Res. 2008;1(2):99–115. doi:10.1007/s12274-008-8018-3.
Ahmed A, Rushworth JV, Hirst NA, Millner PA. Biosensors for whole-cell bacterial detection. Clin Microbiol Rev. 2014;27(3):631–46. doi:10.1128/cmr.00120-13.
Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9(1):247. doi:10.1186/1556-276x-9-247.
Chang AC, Gillespie JB, Tabacco MB. Enhanced detection of live bacteria using a dendrimer thin film in an optical biosensor. Anal Chem. 2001;73(3):467–70.
Ji J, Schanzle J, Tabacco M. Real-time detection of bacterial contamination in dynamic aqueous environments using optical sensors. Anal Chem. 2004;76(5):1411–8. doi:10.1021/ac034914q.
Deisingh A, Thompson M. Biosensors for the detection of bacteria. Can J Microbiol. 2004;50(2):69–77.
Sarkar A, Kaganove S, Dvornic P, Satoh P. Colorimetric biosensors based on polydiacetylene (PDA) and polyamidoamine (PAMAM) dendrimers. Polymer News. 2005;30(12):370–7.
Petit A, Eullaffroyb P, Debenesta T, Gagnéa F. Toxicity of PAMAM dendrimers to Chlamydomonas reinhardtii. Aquat Toxicol. 2010;100(2):187–93. doi:10.1016/j.aquatox.2010.01.019.
Lopez A, Reins R, McDermott A, Trautner B, Cai C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol Biosyst. 2009;5(10):1148–56. doi:10.1039/b904746h.
Lu Y, Slomberg D, Shah A, Schoenfisch M. Nitric oxide-releasing amphiphilic poly(amidoamine) (PAMAM) dendrimers as antibacterial agents. Biomacromolecules. 2013;14(10):3589–98. doi:10.1021/bm400961r.
Zhao J, Jensen L, Sung J, Zou S, Schatz G, Van Duyne R. Interaction of plasmon and molecular resonances for rhodamine 6G adsorbed on silver nanoparticles. Am Chem Soc. 2007;129(24):7647–56.
Ren H, Kulkarni D, Kodiyath R, Xu W, Choi I, Tsukruk V. Competitive adsorption of dopamine and rhodamine 6G on the surface of graphene oxide. Appl Mater Interfaces. 2014;6(4):2459–70. doi:10.1021/am404881p.
Jin S, Xu Z, Chen J, Liang X, Wu J, Qian X. Determination of organophosphate and carbamate pesticides based on enzyme inhibition using a pH-sensitive fluorescence probe. Anal Chim Acta. 2004;523(1):117–23. doi:10.1016/j.aca.2004.05.030.
Rosenthal S, Chang J, Kovtun O, McBride J, Tomlinson I. Biocompatible quantum dots for biological applications. Chem Biol. 2011;18(1):10–24. doi:10.1016/j.chembiol.2010.11.013.
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5(9):763–75. doi:10.1038/nmeth.1248.
Geraldo DA, Duran-Lara EF, Aguayo D, Cachau RE, Tapia J, Esparza R, et al. Supramolecular complexes of quantum dots and a polyamidoamine (PAMAM)-folate derivative for molecular imaging of cancer cells. Anal Bioanal Chem. 2011;400(2):483–92. doi:10.1007/s00216-011-4756-2.
Miao T, Wang Z, Li S, Wang X. Sensitive fluorescent detection of Staphylococcus aureususing nanogold linked CdTe nanocrystals as signal amplification labels. Microchim Acta. 2011;172(3):431–7. doi:10.1007/s00604-010-0505-z.
Gaan S, He G, Feenstra R, Walker J, Towe E. Size, shape, composition, and electronic properties of InAs/GaAs quantum dots by scanning tunneling microscopy and spectroscopy. J Appl Phys. 2010;108(11):1–13. doi:10.1063/1.3518680.
Byrne B, Stack E, Gilmartin N, O'Kennedy R. Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors (Basel). 2009;9(6):4407–45. doi:10.3390/s90604407.
Halford C, Gau V, Churchill BM, Haake DA. Bacterial detection & identification using electrochemical sensors. J Vis Exp. 2013;74:1–8. doi:10.3791/4282.
Tsai H, Hodgson D. Development of a synthetic minimal medium for Listeria monocytogenes. Appl Environ Microbiol. 2003;69(11):6943–5. doi:10.1128/aem.69.11.6943-6945.2003.
Spalding MD, Prigge ST. Lipoic acid metabolism in microbial pathogens. Microbiol Mol Biol Rev. 2010;74(2):200–28.
Christensen QH, Hagar JA, O'Riordan MX, Cronan JE. A complex lipoate utilization pathway in Listeria monocytogenes. J Biol Chem. 2011;286(36):31447–56. doi:10.1074/jbc.M111.273607.
Keeney KM, Stuckey JA, O'Riordan MX. LplA1-dependent utilization of host lipoyl peptides enables Listeria cytosolic growth and virulence. Mol Microbiol. 2007;66(3):758–70. doi:10.1111/j.1365-2958.2007.05956.x.
Wang H, Li Y, Slavic M. Rapid Detection of Listeria monocytogens using quantum dots and nanobeads-based optical biosensor. J Rapid Methods Autom Microbiol. 2007;15(1):67–76. doi:10.1111/j.1745-4581.2007.00075.x.
Sun W, Qi X, Zhang Y, Yang H, Gao H, Chen Y, et al. Electrochemical DNA biosensor for the detection of Listeria monocytogenes with dendritic nanogold and electrochemical reduced graphene modified carbon ionic liquid electrode. Electrochim Acta. 2012;85:145–51. doi:10.1016/j.electacta.2012.07.133.
Davis D, Guo X, Musavi L, Lin C-S, Chen S-H, Wu V. Gold nanoparticle-modified carbon electrode biosensor for the detection of Listeria monocytogenes. Ind Biotechnol. 2013;9(1):31–6. doi:10.1089/ind.2012.0033.
Li L, Qian H, Fang N, Ren J. Significant enhancement of the quantum yield of CdTe nanocrystals synthesized in aqueous phase by controlling the pH and concentrations of precursor solutions. J Lumin. 2006;116(1–2):59–66. doi:10.1016/j.jlumin.2005.03.001.
An LM, Yang YQ, Su WH, Yi J, Liu CX, Chao KF, et al. Enhanced fluorescence from CdTe quantum dots self-assembled on the surface of silver nanoparticles. J Nanosci Nanotechnol. 2010;10(3):2099–103.
Klayman D, Griffin T. Reaction of selenium with sodium borohydride in protic solvents. A facile method for the introduction of selenium into organic molecules. J Am Chem Soc. 1973;95(1):197–99. doi:10.1021/ja00782a034.
Park S, Chibli H, Nadeau J. Solubilization and bio-conjugation of quantum dots and bacterial toxicity assays by growth curve and plate count. J Vis Exp. 2012;65, e3969. doi:10.3791/3969.
Zhao X, Hilliard LR, Mechery SJ, Wang Y, Bagwe RP, Jin S, et al. A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc Natl Acad Sci U S A. 2004;101(42):15027–32. doi:10.1073/pnas.0404806101.
Mesa M, Macías M, Cantero D, Barja F. Use of the direct epifluorescent filter technique for the enumeration of viable and total acetic acid bacteria from vinegar fermentation. J Fluoresc. 2003;13(3):261–5. doi:10.1023/A:1025094017265.
Maturin L, Peeler J. Laboratory methods - BAM: aerobic plate count. US Food and Drug Administration, Silver Spring. http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm063346.htm (2001). Accessed 4 Apr 16.
Vogelgesang J, Hädrich J. Limits of detection, identification and determination: a statistical approach for practitioners. Accred Qual Assur. 1998;3(6):242–55. doi:10.1007/s007690050234.
Wang C, Yan Q, Liu H-B, Zhou X-H, Xiao S-J. Different EDC/NHS activation mechanisms between PAA and PMAA brushes and the following amidation reactions. Langmuir. 2011;27(19):12058–68. doi:10.1021/la202267p.
Sun L, Yu X, Sun M, Wang H, Xu S, Dixon JD, et al. Preparation of quantum dots encoded microspheres by electrospray for the detection of biomolecules. J Colloid Interface Sci. 2011;358(1):73–80. doi:10.1016/j.jcis.2011.02.047.
Guterres S, Beck R, Pohlmann A. Spray-drying technique to prepare innovative nanoparticulated formulations for drug administration: a brief overview. Braz J Phys. 2009;39(1A):205–9. doi:10.1590/S0103-97332009000200013.
Zhou Z, Lin M, Chen Z, Sun H, Zhang H, Sun H, et al. Simple synthesis of highly luminescent water-soluble CdTe quantum dots with controllable surface functionality. Chem Mater. 2011;23(21):4857–62. doi:10.1021/cm202368w.
Forry SP, Madonna MC, López-Pérez D, Lin NJ, Pasco MD. Automation of antimicrobial activity screening. AMB Express. 2016;6:1–10. doi:10.1186/s13568-016-0191-2.
Wagner M, McLauchlin J. Biology. In: Liu D, editor. Handbook of Listeria monocytogenes. Boca Raton: CRC Press; 2008.
Heigl N, Bachmann S, Petter CH, Marchetti-Deschmann M, Allmaier G, Bonn GK, et al. Near-infrared spectroscopic study on guest-host interactions among G0-G7 amine-terminated poly(amidoamine) dendrimers and porous silica materials for simultaneously determining the molecular weight and particle diameter by multivariate calibration techniques. Anal Chem. 2009;81(14):5655–62. doi:10.1021/ac900375z.
Tmejova K, Hynek D, Kopel P, Gumulec J, Krizkova S, Guran R, et al. Structural effects and nanoparticle size are essential for quantum dots-metallothionein complex formation. Colloids Surf B. 2015;134:262–72. doi:10.1016/j.colsurfb.2015.06.045.
Dey D, Goswami T. Optical biosensors: a revolution towards quantum nanoscale electronics device fabrication. J Biomed Biotechnol. 2011;2011:348218. doi:10.1155/2011/348218.
Pohlmann C, Humenik M, Sprinzl M. Detection of bacterial 16S rRNA using multivalent dendrimer-reporter enzyme conjugates. Biosens Bioelectron. 2009;24(11):3383–6. doi:10.1016/j.bios.2009.04.017.
Mandal TK, Parvin N. Rapid detection of bacteria by carbon quantum dots. J Biomed Nanotechnol. 2011;7(6):846–8.
Dumas EM, Ozenne V, Mielke RE, Nadeau JL. Toxicity of CdTe quantum dots in bacterial strains. IEEE Trans Nanobioscience. 2009;8(1):58–64. doi:10.1109/tnb.2009.2017313.
Gonzalo S, Rodea-Palomares I, Leganes F, Garcia-Calvo E, Rosal R, Fernandez-Pinas F. First evidences of PAMAM dendrimer internalization in microorganisms of environmental relevance: a linkage with toxicity and oxidative stress. Nanotoxicology. 2015;9(6):706–18. doi:10.3109/17435390.2014.969345.
Jain K, Kesharwani P, Gupta U, Jain NK. Dendrimer toxicity: let's meet the challenge. Int J Pharm. 2010;394(1-2):122–42. doi:10.1016/j.ijpharm.2010.04.027.
Kloepfer JA, Mielke RE, Nadeau JL. Uptake of CdSe and CdSe/ZnS Quantum dots into bacteria via purine-dependent mechanisms. Appl Environ Microbiol. 2005;71(5):2548–57. doi:10.1128/aem.71.5.2548-2557.2005.
Mahendra S, Zhu H, Colvin VL, Alvarez PJ. Quantum dot weathering results in microbial toxicity. Environ Sci Technol. 2008;42(24):9424–30.
Jin T, Sun D, Su JY, Zhang H, Sue HJ. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157:H7. J Food Sci. 2009;74(1):M46–52. doi:10.1111/j.1750-3841.2008.01013.x.
Tyagi A, Rawat K, Verma A, Bohidar H. Mechanistic evaluation of the size dependent antimicrobial activity of water soluble QDs. Anal Methods. 2015;8(5):1060–8. doi:10.1039/C5AY02742J.
Lin S, Bhattacharya P, Rajapakse N, Brune D, Ke PC. Effects of quantum dots adsorption on algal photosynthesis. J Phys Chem C. 2009;113(25):10962–6. doi:10.1021/jp904343s.
Swift BJF, Baneyx F. Microbial uptake, toxicity, and fate of biofabricated ZnS:Mn nanocrystals. PLoS One. 2015;10(4):1–14. doi:10.1371/journal.pone.0124916.
Bierne H, Cossart P. Listeria monocytogenes surface proteins: from genome predictions to function. Microbiol Mol Biol Rev. 2007;71(2):377–97. doi:10.1128/mmbr.00039-06.
Hirschey MD, Han YJ, Stucky GD, Butler A. Imaging Escherichia coli using functionalized core/shell CdSe/CdS quantum dots. J Biol Inorg Chem. 2006;11(5):663–9. doi:10.1007/s00775-006-0116-7.
Pettipher GL, Mansell R, McKinnon CH, Cousins CM. Rapid membrane filtration-epifluorescent microscopy technique for direct enumeration of bacteria in raw milk. Appl Environ Microbiol. 1980;39(2):423–9.
Tortorello ML, Stewart DS. Antibody-direct epifluorescent filter technique for rapid, direct enumeration of Escherichia coli O157:H7 in beef. Appl Environ Microbiol. 1994;60(10):3553–9.
Zemser R, Martin S. Heat stability of virulence-associated enzymes from Listeria monocytogenes SLCC 5764. J Food Prot. 1998;61(7):899–902.
Acknowledgement
L.G. and L.S.S. thank FONDECYT (FONDECYT Initiation no. 11150390 and Regular no. 1140642). Additional financial support from PIEI (QUI-BIO) from Universidad de Talca is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributions
L.S.S. and W.D. conceived and designed the experiments; R.I.C., L.G., F.M.N., and Z.L.C. performed research and analyzed the data. All authors analyzed and interpreted data, drafted the paper, and read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Donoso, W., Castro, R.I., Guzmán, L. et al. Fast detection of Listeria monocytogenes through a nanohybrid quantum dot complex. Anal Bioanal Chem 409, 5359–5371 (2017). https://doi.org/10.1007/s00216-017-0481-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0481-9