Abstract
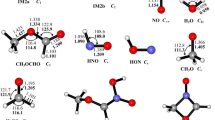
The mechanisms of CH2SH with NO2 reaction were investigated on the singlet and triplet potential energy surfaces (PES) at the BMC-CCSD//B3LYP/6-311 + G(d,p) level. The result shows that the title reaction is more favourable on the singlet PES thermodynamically, and it is less competitive on the triplet PES. On the singlet PES, the initial addition of CH2SH with NO2 leads to HSCH2NO2 (IM2) without any transition state, followed by a concerted step involving C–N fission and shift of H atom from S to O giving out CH2S + trans-HONO, which is the major products of the title reaction. With higher barrier height, the minor products are CH2S + HNO2, formed by a similar concerted step from the initial adduct HSCH2ONO (IM1). The direct abstraction route of H atom in SH group abstracted by O atom might be of some importance. It starts from the addition of the reactants to form a weak interaction molecular complex (MC3), subsequently, surmounts a low barrier height leading to another complex (MC2), which gives out CH2S + trans-HONO finally. Other direct hydrogen abstraction channels could be negligible with higher barrier heights and less stable products.





Similar content being viewed by others
References
Atkinson R, JN Pitts Jr, Aschrmann SM (1984) J Phys Chem 88:1584
Yin F, Grosjean D, Seinfeld JH (1990) J Atmos Chem 11:309
Wine PH, Kreutter NM, Gump CA, Ravishankara AR (1981) J Phys Chem 85:2660
Tyndall GS, Ravishankara AR (1989) J Phys Chem 93:2426
Martinez E, Albaladejo J (2000) Atmos Environ 34:5295
Wang SK, Zhang QZ, Zhou JH, Gu YS (2004) Acta Chimica Sinica 62:550
Martinez E, Albaladejo J (1999) Chem Phys Lett 308:37
Chang PF, Wang TT, Wang NS (2000) J Phys Chem A 104:5525
Tang YZ, Sun H, Pan YR, Wang RS (2007) Int J Quantum Chem 107:1495
Anastasi C, Broomfield M (1992) J Phys Chem 96:696
Zhang JX, Li ZS, Liu JY, Sun CC (2006) J Phys Chem A 110:2690
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 03; Gaussian, Pittsburgh PA
Curtiss LA, Raghavachari K, Redfern PC, Pople JA (1998) J Chem Phys 109:7764
Baboul AG, Curtiss LA, Raghavachari K (1999) J Chem Phys 110:7650
Fast PL, Corchado JC, Truhlar DG (1999) J Phys Chem A 103:5129
Lynch BJ, Zhao Y, Truhlar DG (1999) J Phys Chem A 109:1643
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 20773021) and the Science Foundation for Young Teachers of Northeast Normal University (No. 20070315). We are greatly thankful for the referees’ helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, YZ., Pan, YR., He, B. et al. Theoretical study on the reaction mechanism of CH2SH + NO2 . Theor Chem Account 122, 67–76 (2009). https://doi.org/10.1007/s00214-008-0485-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-008-0485-9