Abstract
Though anti-metastatic function of non-metastatic 2 (NME2) has been implicated in multiple cancers, mechanisms of metastases control by NME2 are not clearly understood. Recent observations indicating the involvement of telomerase, the ribonucleoprotein required for telomere synthesis, in metastatic outcome are interesting. Notably, though the role of telomerase dysfunction in tumorigenesis is relatively well studied, involvement in metastasis progression is poorly understood. Recent findings demonstrate NME2 presence at telomere ends, association with telomerase, and NME2’s role in inhibition of telomerase activity in cancer cells. These present a novel opportunity to investigate mechanisms underlying NME2-mediated metastasis suppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Role of non-metastatic 2 in suppression of cancer spread is poorly understood
The non-metastatic factor 23 (Nm23) human isoform 2 (H2) also known as non-metastatic 2 (NME2) belongs to the non-metastatic 23 gene family—the NME2 gene codes for a 152-amino acid protein (∼18 kD) and is located on chromosome 17q21.3. Multiple functions are reported for NME2 including histidine kinase enzymatic activity, association with proteins where the enzymatic function of NME2 is not apparent and, interestingly, NME2 was also found to influence gene expression (Desvignes et al., 2009; Mehta and Orchard, 2009). Regulation of various cellular processes in normal and/or cancer cells, such as invasiveness and drug resistance by NME2 have been studied (Hippe et al., 2003; Srivastava et al., 2006a; Di et al., 2010a; Thakur et al., 2015; Yadav et al. 2014) and in some cases, demonstrated in rodent and zebra fish (Desvignes et al., 2011; Boissan and Lacombe, 2011; Wieland, 2007). Role in transcription was implicated on identification of NME2 as a purine nucleotide binding factor involved in expression of the proto-oncogene c-MYC (Berberich and Postel, 1995; Hildebrandt et al., 1995; Postel et al., 1993; Postel and Ferrone, 1994). Further work using chromatin-immunoprecipitation studies demonstrated NME2 physically occupies the c-MYC promoter within cells (Thakur et al., 2009). In addition, NME2 was found in the OCA-S multi-subunit co-activator complex present on the histone 2B promoter (Zheng et al., 2003); NME2-dependent histidine kinase activity in human cells was a key factor in phosphorylation of the KCa3.1 ion channel in CD4+ T lymphocytes; mice devoid of NME2 (knockout) had impaired KCa3.1 function (Di et al., 2010b; Srivastava et al., 2006b); and a ternary complex comprising NME2, ICAP1-alpha, and Lbc was observed to oppose cell migration (Iwashita et al., 2004). Moreover, the role of NME1 as a close homologue was reported in DNA damage and genome stability—studies by Kaetzel et al. found cells with a mutant form of NME1 devoid of 3ʹ-5ʹ exonuclease activity had altered metastatic proclivity compared to wild-type cells (Kaetzel et al., 2006a). Consistent with this observation, treatment of cells with DNA-damaging agents resulted in increased nuclear translocation of NME1 suggesting a possible role in DNA repair (Novak et al., 2011; Kaetzel et al., 2006b). Similar observations were reported in the yeast, Saccharomyces cerevisiae where ablation of the NME homologue YNK1 delayed DNA damage response (Yang et al., 2009).
Although a number of different roles of NME2 have been described, somewhat surprisingly given the clinical implications, mechanisms of NME2 and metastasis suppression remains poorly understood. Particularly, functions of NME2 with potential to control aggressive growth of cancer cells needs further study (Thakur et al., 2011). Herein we focus on an emerging aspect where NME2-mediated metastases suppression could involve the human ribonucleoprotein telomerase.
Telomere and telomerase: connection with tumor cells
Although cytogenetic studies in maize by Barbara McClintock in 1931 first noted the “natural ends” of chromosome, these were termed as telomere by Hermann Muller, and independently by J.B.S. Haldane in 1938 (reviewed in (Blackburn et al., 2006)). Interestingly, the importance of telomeres was fully realized when it became evident that replication of telomeric DNA by conventional DNA polymerases was not possible (Blackburn, 1991). Two major lines of thought emerged to address the replication problem associated with telomere elongation. It was proposed to rely either on recombination events between telomeres or on the existence of a novel enzymatic activity that could synthesize telomere repeats de novo. Discovery of the ribonucleo-protein complex telomerase comprising reverse transcriptase (TERT (Greider and Blackburn, 1985)) and RNA motif (TERC (Chen and Greider, 2004; Lingner and Cech, 1996)) with DNA polymerase activity at telomeres supported the second point. On the other the hand, in some tumor cell lines, a recombination-based mechanism was found to maintain and elongate telomeres in the absence of telomerase—this was termed as alternative lengthening of telomeres (ALT) (Bryan et al., 1997).
Interestingly though, most eukaryotes use telomerase to maintain telomeres (Greider and Blackburn, 1987) adult somatic tissues do not have sufficient telomerase activity (Collins and Mitchell, 2002) and, therefore, accumulate chromosomal damage at telomeres with age (Herbig et al., 2006) (Blasco, 2007; Harley et al., 1992). In contrast, activation of the telomerase gene in many tumors results in maintenance and/or in some cases, elongation of telomeres thereby contributing to cell proliferation and tumorigenicity (Shay and Wright, 2006) (Blasco, 2007).
Telomeres, telomerase and cancer spread (metastasis)
Though genomic instability due to altered telomere length and telomerase reactivation are now established steps in development and maintenance of tumor cells (Artandi et al., 2000) (Artandi and Depinho, 2010), the connection between telomerase activity/telomere length and metastasis (spread of tumor cells to distant secondary organ sites) is not clearly understood. One of the earliest correlations was from Griffith et al.: reduced telomeric DNA content was found to correlate with genomic instability and metastasis in invasive human breast carcinoma (Griffith et al., 1999). In a recent study, authors examined head and neck squamous cell carcinoma (HNSCC) cells in a telomerase-deficient (made by genetically knocking out Terc, the RNA component of telomerase) murine background with either long or short telomeres—G1 Terc−/− telomerase-deficient mice with long telomeres, compared to G5 Terc−/− telomerase-deficient mice with short telomeres, had fewer lymph node metastases (Bojovic and Crowe, 2011). Interestingly, authors also noted the initial loss of telomerase activity in primary HNSCC resulted in reduced metastasis (that is in G1 Terc−/− mice) whereas during later generations, short telomeres (due to lack of telomerase activity) promoted metastasis in G5 Terc−/− mice. Another study from the same group characterized neu proto-oncogene driven mammary tumor formation in G1 Terc−/− and G3 Terc−/− (telomerase deficient with short telomeres), and Terc+/+ mice. Like the earlier study, short telomeres dramatically increased lung metastasis (Bojovic and Crowe, 2013).
Direct correlation between telomerase activity, hTERT levels and cellular invasion was found when ribozyme-mediated suppression of telomerase RNA (TER) levels in melanoma cells resulted in reduced telomerase activity and decreased invasive potential of melanoma cells (Bagheri et al., 2006). Furthermore, it was observed that TER suppression results in down-regulation of the glycolytic pathway and reduced glucose metabolism provided a possible basis for reduced metastatic potential. Consistent with this, Yu et al. reported introduction of the full-length complementary DNA (cDNA) of human telomerase gene hTERT into a telomerase-negative osteosarcoma cell line U2OS (hTERT/U2OS) increased invasive ability of cells. As expected, telomere length in hTERT/U2OS cells was longer than that in vector control or un-transfected U2OS cells (Yu et al., 2009).
In addition, a large pool of clinical data became available where telomerase level correlated with progression of several types of cancer including acute leukemia, breast, prostate, lung and melanoma (Artandi and Depinho, 2010). A recent meta-analysis of Lu et al. revealed high telomerase activity was associated with the presence of lymph node metastasis, depth of invasion, distant metastasis, tumor size, and metastatic stage in gastric cancer (Lu et al., 2012). Another study reported 63 of 71 breast cancer tissues with telomerase activity (88.7 %) while no telomerase activity was detected in their paired normal tissues (Rha et al., 1999); interestingly, telomerase activity correlated with node metastasis and stage, but not tumor size or hormonal status of receptors. Comparison of telomerase activity and hTERT mRNA (hTERT) expression in cancerous and non-cancerous lung tissues of 62 lung cancer patients also revealed high telomerase activity and hTERT levels in cancer tissues relative to non-cancerous tissues (Hara et al., 2001). Furthermore, both telomerase activity and hTERT expression significantly correlated with lymph node metastasis (P < 0.05). This study also noted that patients with hTERT-positive tumor survived for a significantly shorter period than those with hTERT-negative tumor (P = 0.0334) (Hara et al., 2001).
Taken together, an apparent contrast emerges. Loss of telomerase activity promoted metastasis in breast and head and neck cancer where absence of the telomerase RNA component led to catalytically inactive telomerase (Terc−/− mice). Whereas other studies, including clinical data, suggest low telomerase activity imparts decreased metastatic potential. These suggest a clear need for further work to understand underlying mechanisms that govern links between telomerase levels or activity and metastatic outcomes.
NME2-mediated metastasis—emerging connection with telomerase function
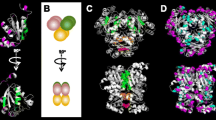
Given the complexity noted in studies discussed above newly observed NME2 function and its impact on telomerase activity are of interest. There are several reports related to involvement of NME2 in metastasis (Bodey et al., 1997; Miyazaki et al., 1999; Steeg et al., 1988; Thakur et al., 2011). NME2 expression was found to negatively correlate with advanced/metastatic stages across several tumor types (Thakur et al., 2011). Surprisingly, analysis of short sequence reads from chromatin immunoprecipitation of NME2 in A549 cells followed by massively parallel chromatin immunoprecipitation sequencing (ChIP-seq) revealed a large number of sequences from telomere ends (Kar et al., 2012). We found support for this unanticipated finding from earlier work reporting in vitro association of NME2 with oligonucleotide sequences representing human telomere ends (Nosaka et al., 1998). The possibility of NME2 being involved in direct/indirect association with telomere ends therefore presents a novel opportunity to understand NME2 function in the context of metastasis (Fig. 1).
Intracellular association of NME2 and telomeric DNA was clearly observed using chromatin immunoprecipitation (ChIP) and experiments; co-immunoprecipitation experiments further suggested physical interaction between NME2 and telomerase in metastatic cancer cells (Kar et al., 2012). Furthermore, direct physical association was observed using purified hTERT and NME2 (Srivastava et al., 2014 unpublished). Earlier studies suggested telomerase levels impact invasiveness of tumors (discussed earlier); therefore, reported involvement of NME2 in metastasis prompted questions about role of NME2 with regard to telomerase activity. Notably, NME2 was found to not only associate with telomerase but also inhibit telomerase activity in vitro as well as in cellulo (Kar et al., 2012). In addition, it was also observed that inhibition of telomerase activity in cells expressing elevated levels of NME2 resulted in decrease in telomere length (Fig. 2).
Stable expression of NME2 leads to reduced telomerase activity and telomere shortening. RT-PCR (a) and western blot (b) for stable expression of GFP-tagged NME2 in MDAM B-231 cells. c Telomerase activity in GFP-NME2 cells using real time quantification of telomerase activity; *P < 0.05. d Southern blot analysis of stable GFP-NME2 MDAMB-231 cells to check telomere length compared to vector transformed cells
Conclusion—a potential mechanistic model for further work
Keeping in mind the poorly understood link between telomerase (level and activity) and metastatic proclivity of human cancer cells on one hand, NME2 functions noted in metastasis control (Thakur et al., 2015; Yadav et al. 2014) on the other hand, the emerging axis between NME2 and telomerase activity is interesting. Though results noted above suggest the role of NME2 in the suppression of telomerase activity, further work is required to understand whether and how this impacts metastasis. Based on current findings, a possible model can be envisaged where NME2-telomerase interaction impacts telomerase both catalytically and non-catalytically. Whereas decreased catalytic activity is bound to influence telomere length directly resulting reduction in metastatic potential is also possible as reported in some tumors (Bagheri et al., 2006; Saito et al., 1997). Somewhat surprisingly, a recent finding showed extra-telomeric binding of telomerase at gene promoters. Authors further demonstrated that resultant activation wnt-beta catenin genes increased the aggressiveness of cells suggesting a non-catalytic function of telomerase that impacts metastasis (Park et al., 2009). Considering the role of NME2 in this context is interesting: NME2-telomerase association and its effect, if any, on telomerase-mediated activation of wnt-beta catenin signaling could be a possible mode of influencing aggressive potential of cells. Further work that tests these possibilities, particularly the underlying mechanisms in the light of multiple reported functions of NME2 like histidine kinase activity, transcription regulation and role in DNA damage, is therefore expected to impact our understanding of metastasis in important ways.
Material and method
RT-PCR for hTERT and NME2
RNA was extracted using TRIzol reagent (Sigma, USA) as per manufacturer protocol. Complementary DNA was synthesized using cDNA synthesis kit (Applied Biosystems, GMBH) following the manufacturer’s instructions. Transcript levels were determined using the following primer set.
hTERT: fwd-GCCGATTGTGAACATGGACTACG,
rev-GCTCGTAGTTGAGCACGCTGAA.
Beta-actin: fwd-TGCGTGACATTAAGGAGAAG,
rev-CTGCATCCTGTCGGCAATG.
Antibodies and western blotting
For western analysis, cell lysate were prepared using 1X Cell culture lysis reagent (Promega) and were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Immobilon FL, Millipore); following primary and secondary antibodies were used for immuno-blotting. Primary antibodies anti-GFP antibody and secondary antibodies anti-mouse alkaline phosphates conjugated were from Sigma.
Analysis of telomerase activity
Cells were lysed in a lysis buffer and telomerase-containing fraction was prepared for real time telomerase activity assay using quantitative telomerase detection kit (US Biomax, USA) according to the manufacturer’s protocol. For stable cells with sustained NME2 or empty vector expression (control cells) equal amount of lysate was taken and further used for the assay as mentioned above. All assays were performed in triplicate and relative fold-change in expression was calculated from observed Ct values.
Terminal restriction fragment length analysis
Measurements of telomere lengths were done as described previously (van, SB. and de, LT 1997; Aubert et al., 2012). Briefly, DNA was isolated and was digested to completion with multiple restriction enzyme mix (∼1 U/μg each of HinfI and RsaI, Roche Boehringer Mannheim, Indianapolis, IN). The digested DNA was separated on a 0.7 % agarose gel in 0.5X Tris-borate EDTA [0.5 mol/L Tris-borate (pH 8.3), 10 mmol/L EDTA]. The gel was denatured for 20 min in 0.5 mol/L NaOH/1.5 mol/L NaCl, rinsed with distilled H2O for 10 min, dried on Whatman No. 3MM paper under vacuum for 1 h at 55 °C, and neutralized for 15 min in 1.5 mol/L NaCl, 0.5 mol/L Tris–HCl (pH 8.0). The gel was probed with a radio-labeled telomeric probe for 16 h at 42 °C in 5X SSC buffer, 5X Denhardt’s solution, 10 mmol/L Na2HPO4, and 1 mmol/L Na2H2P2O7. The gel was then washed once for 20 min in 2X SSC, twice for 15 min each in 0.1X SSC at room temperature, and exposed to a phosphor screen (PhosphorImager).
Invasion assay
A modified version of the Boyden chamber assay (Cell bio Labs CytoSelect™) was used to determine the invasion potential of cells as per manufacturer’s instructions (also described earlier (Thakur et al., 2015)). Briefly, following suspension in serum-free media (∼half-million cells/ml) cells were incubated (24 h) for invasion assays in chambers pre-coated with specialized membrane comprising constituents of the extracellular matrix purified from mouse sarcoma tissue.
References
Artandi SE, Depinho RA (2010) Telomeres and telomerase in cancer. Carcinogenesis 31:9–18
Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, Depinho RA (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406:641–645
Aubert G, Hills M, Lansdorp PM (2012) Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res 730:59–67
Bagheri S, Nosrati M, Li S, Fong S, Torabian S, Rangel J, Moore DH, Federman S, Laposa RR, Baehner FL, Sagebiel RW, Cleaver JE, Haqq C, Debs RJ, Blackburn EH, Kashani-Sabet M (2006) Genes and pathways downstream of telomerase in melanoma metastasis. Proc Natl Acad Sci U S A 103:11306–11311
Berberich SJ, Postel EH (1995) PuF/NM23-H2/NDPK-B transactivates a human c-myc promoter-CAT gene via a functional nuclease hypersensitive element. Oncogene 10:2343–2347
Blackburn EH (1991) Structure and function of telomeres. Nature 350:569–573
Blackburn EH, Greider CW, Szostak JW (2006) Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 12:1133–1138
Blasco MA (2007) Telomere length, stem cells and aging. Nat Chem Biol 3:640–649
Bodey B, Bodey B Jr, Groger AM, Siegel SE, Kaiser HE (1997) Nm23/nucleoside diphosphate (NDP) kinase expression in human malignant melanomas: significance and implications in tumor biology. Anticancer Res 17:505–511
Boissan M, Lacombe ML (2011) Learning about the functions of NME/NM23: lessons from knockout mice to silencing strategies. Naunyn Schmiedebergs Arch Pharmacol 384:421–431
Bojovic B, Crowe DL (2011) Telomere dysfunction promotes metastasis in a TERC null mouse model of head and neck cancer. Mol Cancer Res 9:901–913
Bojovic B, Crowe DL (2013) Dysfunctional telomeres promote genomic instability and metastasis in the absence of telomerase activity in oncogene induced mammary cancer. Mol Carcinog 52:103–117
Bryan TM, Englezou A, la-Pozza L, Dunham MA, Reddel RR (1997) Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med 3:1271–1274
Chen JL, Greider CW (2004) An emerging consensus for telomerase RNA structure. Proc Natl Acad Sci U S A 101:14683–14684
Collins K, Mitchell JR (2002) Telomerase in the human organism. Oncogene 21:564–579
Desvignes T, Pontarotti P, Fauvel C, Bobe J (2009) Nme protein family evolutionary history, a vertebrate perspective. BMC Evol Biol 9:256. doi:10.1186/1471-2148-9-256., 256-259
Desvignes T, Fauvel C, Bobe J (2011) The NME gene family in zebrafish oogenesis and early development. Naunyn Schmiedebergs Arch Pharmacol 384:439–449
Di L, Srivastava S, Zhdanova O, Sun Y, Li Z, Skolnik EY (2010a) Nucleoside diphosphate kinase B knock-out mice have impaired activation of the K+ channel KCa3.1, resulting in defective T cell activation. J Biol Chem 285:38765–38771
Di L, Srivastava S, Zhdanova O, Sun Y, Li Z, Skolnik EY (2010b) Nucleoside diphosphate kinase B knock-out mice have impaired activation of the K+ channel KCa3.1, resulting in defective T cell activation. J Biol Chem 285:38765–38771
Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405–413
Greider CW, Blackburn EH (1987) The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51:887–898
Griffith JK, Bryant JE, Fordyce CA, Gilliland FD, Joste NE, Moyzis RK (1999) Reduced telomere DNA content is correlated with genomic instability and metastasis in invasive human breast carcinoma. Breast Cancer Res Treat 54:59–64
Hara H, Yamashita K, Shinada J, Yoshimura H, Kameya T (2001) Clinicopathologic significance of telomerase activity and hTERT mRNA expression in non-small cell lung cancer. Lung Cancer 34:219–226
Harley CB, Vaziri H, Counter CM, Allsopp RC (1992) The telomere hypothesis of cellular aging. Exp Gerontol 27:375–382
Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM (2006) Cellular senescence in aging primates. Science 311:1257
Hildebrandt M, Lacombe ML, Mesnildrey S, Veron M (1995) A human NDP-kinase B specifically binds single-stranded poly-pyrimidine sequences. Nucleic Acids Res 23:3858–3864
Hippe HJ, Lutz S, Cuello F, Knorr K, Vogt A, Jakobs KH, Wieland T, Niroomand F (2003) Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and Gbeta subunits. Specific activation of Gsalpha by an NDPK B.Gbetagamma complex in H10 cells. J Biol Chem 278:7227–7233
Iwashita S, Fujii M, Mukai H, Ono Y, Miyamoto M (2004) Lbc proto-oncogene product binds to and could be negatively regulated by metastasis suppressor nm23-H2. Biochem Biophys Res Commun 320:1063–1068
Kaetzel DM, Zhang Q, Yang M, McCorkle JR, Ma D, Craven RJ (2006a) Potential roles of 3'-5' exonuclease activity of NM23-H1 in DNA repair and malignant progression. J Bioenerg Biomembr 38:163–167
Kaetzel DM, Zhang Q, Yang M, McCorkle JR, Ma D, Craven RJ (2006b) Potential roles of 3'-5' exonuclease activity of NM23-H1 in DNA repair and malignant progression. J Bioenerg Biomembr 38:163–167
Kar A, Saha D, Purohit G, Singh A, Kumar P, Yadav VK, Kumar P, Thakur RK, Chowdhury S (2012) Metastases suppressor NME2 associates with telomere ends and telomerase and reduces telomerase activity within cells. Nucleic Acids Res 40:2554–2565
Lingner J, Cech TR (1996) Purification of telomerase from Euplotes aediculatus: requirement of a primer 3' overhang. Proc Natl Acad Sci U S A 93:10712–10717
Lu MH, Deng JQ, Cao YL, Fang DC, Zhang Y, Yang SM (2012) Prognostic role of telomerase activity in gastric adenocarcinoma: A meta-analysis. Exp Ther Med 3:728–734
Mehta A, Orchard S (2009) Nucleoside diphosphate kinase (NDPK, NM23, AWD): recent regulatory advances in endocytosis, metastasis, psoriasis, insulin release, fetal erythroid lineage and heart failure; translational medicine exemplified. Mol Cell Biochem 329:3–15
Miyazaki H, Fukuda M, Ishijima Y, Takagi Y, Iimura T, Negishi A, Hirayama R, Ishikawa N, Amagasa T, Kimura N (1999) Overexpression of nm23-H2/NDP kinase B in a human oral squamous cell carcinoma cell line results in reduced metastasis, differentiated phenotype in the metastatic site, and growth factor-independent proliferative activity in culture. Clin Cancer Res 5:4301–4307
Nosaka K, Kawahara M, Masuda M, Satomi Y, Nishino H (1998) Association of nucleoside diphosphate kinase nm23-H2 with human telomeres. Biochem Biophys Res Commun 243:342–348
Novak M, Jarrett SG, McCorkle JR, Mellon I, Kaetzel DM (2011) Multiple mechanisms underlie metastasis suppressor function of NM23-H1 in melanoma. Naunyn Schmiedebergs Arch Pharmacol 384:433–438
Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, McLaughlin M, Veenstra TD, Nusse R, McCrea PD, Artandi SE (2009) Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460:66–72
Postel EH, Ferrone CA (1994) Nucleoside diphosphate kinase enzyme activity of NM23-H2/PuF is not required for its DNA binding and in vitro transcriptional functions. J Biol Chem 269:8627–8630
Postel EH, Berberich SJ, Flint SJ, Ferrone CA (1993) Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science 261:478–480
Rha SY, Park KH, Kim TS, Yoo NC, Yang WI, Roh JK, Min JS, Lee KS, Kim BS, Choi JH, Lim HY, Chung HC (1999) Changes of telomerase and telomere lengths in paired normal and cancer tissues of breast. Int J Oncol 15:839–845
Saito Y, Kosugi S, Suda T, Wakabayashi Y, Mishima Y, Hatakeyama K, Kominami R (1997) Telomerase activity and metastasis: expansion of cells having higher telomerase activity within culture lines and tumor tissues. Jpn J Cancer Res 88:732–737
Shay JW, Wright WE (2006) Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discov 5:577–584
Srivastava S, Li Z, Ko K, Choudhury P, Albaqumi M, Johnson AK, Yan Y, Backer JM, Unutmaz D, Coetzee WA, Skolnik EY (2006a) Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol Cell 24:665–675
Srivastava S, Li Z, Ko K, Choudhury P, Albaqumi M, Johnson AK, Yan Y, Backer JM, Unutmaz D, Coetzee WA, Skolnik EY (2006b) Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol Cell 24:665–675
Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, Sobel ME (1988) Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 80:200–204
Thakur RK, Kumar P, Halder K, Verma A, Kar A, Parent JL, Basundra R, Kumar A, Chowdhury S (2009) Metastases suppressor NM23-H2 interaction with G-quadruplex DNA within c-MYC promoter nuclease hypersensitive element induces c-MYC expression. Nucleic Acids Res 37:172–183
Thakur RK, Yadav VK, Kumar P, Chowdhury S (2011) Mechanisms of non-metastatic 2 (NME2)-mediated control of metastasis across tumor types. Naunyn Schmiedebergs Arch Pharmacol 384:397–406
Thakur RK, Yadav VK, Kumar A, Singh A, Pal K, Hoeppner L, Saha D, Purohit G, Basundra R, Kar A, Halder R, Kumar P, Baral A, Kumar MM, Baldi A, Vincenzi B, Lorenzon L, Banerjee R, Kumar P, Shridhar V, Mukhopadhyay D, Chowdhury S (2015) Non-metastatic 2 (NME2)-mediated suppression of lung cancer metastasis involves transcriptional regulation of key cell adhesion factor vinculin. Nucleic Acids Res 42:11589–11600
van, SB. and de, LT (1997). Control of telomere length by the human telomeric protein TRF1. Nature. %20;385, 740-743
Wieland T (2007) Interaction of nucleoside diphosphate kinase B with heterotrimeric G protein betagamma dimers: consequences on G protein activation and stability. Naunyn Schmiedebergs Arch Pharmacol 374:373–383
Yadav VK, Kumar A, Mann A, Aggarwal S, Kumar M, Roy SD, Pore SK, Banerjee R, Mahesh Kumar J, Thakur RK, Chowdhury S (2014) Engineered reversal of drug resistance in cancer cells-metastases suppressor factors as change agents. Nucleic Acids Res 42:764–773
Yang M, Jarrett SG, Craven R, Kaetzel DM (2009) YNK1, the yeast homolog of human metastasis suppressor NM23, is required for repair of UV radiation- and etoposide-induced DNA damage. Mutat Res 660:74–78
Yu ST, Chen L, Wang HJ, Tang XD, Fang DC, Yang SM (2009) hTERT promotes the invasion of telomerase-negative tumor cells in vitro. Int J Oncol 35:329–336
Zheng L, Roeder RG, Luo Y (2003) S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 114:255–266
Acknowledgments
Authors acknowledge Dhurjhoti Saha, Gunjan Purohit, Ankita Singh, Sumitabho Deb Roy, Maneesh Kumar, Vivek Srivastava, and other members of the Chowdhury Lab, particularly, Ramkrishna Thakur for help with editing. Wellcome Trust DBT India Alliance and CSIR, Government of India for research funding. SC is a Senior Fellow of the Wellcome Trust DBT India Alliance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kar, A., Chowdhury, S. Inhibition of telomerase activity by NME2: impact on metastasis suppression?. Naunyn-Schmiedeberg's Arch Pharmacol 388, 235–241 (2015). https://doi.org/10.1007/s00210-014-1077-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1077-y