Abstract
Summary
Osteoporosis is linked to age-related decline of melatonin production; however, the direct effects of melatonin on osteoclastogenesis remain unknown. Our study demonstrates that melatonin at pharmacological concentrations, rather than at physiological concentrations, significantly inhibits osteoclastogenesis. Melatonin-mediated anti-osteoclastogenesis involves a reactive oxygen species (ROS)-mediated but not a silent information regulator type 1 (SIRT1)-independent pathway.
Introduction
Osteoporosis is a bone disorder linked to impaired bone formation and excessive bone resorption. Melatonin has been suggested to treat osteoporosis due to its beneficial actions on osteoblast differentiation. However, the direct effects of melatonin on osteoclastogenesis in bone marrow monocytes (BMMs) remain unknown. This study was to investigate whether melatonin at either physiological or pharmacological concentrations could affect osteoclast differentiation.
Methods
Primary BMMs were isolated from the femurs and tibias of C57BL/6 mice and were induced toward multinucleated osteoclasts, in the presence of melatonin at either physiological (0.01 to 10 nM) or pharmacological (1 to 100 μM) concentrations. Tartrate-resistant acid phosphatase (TRAP) staining was used to label multinucleated osteoclasts and the levels of osteoclast-specific genes were evaluated. To further explore the underlying mechanisms, the roles of silent information regulator type 1 (SIRT1) and reactive oxygen species (ROS) were evaluated.
Results
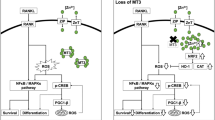
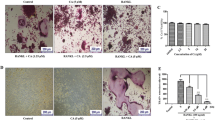
We found that melatonin at pharmacological concentrations, rather than at physiological concentrations, significantly inhibited osteoclast formation in a dose-dependent manner. The number of TRAP-positive cells and the gene expression of osteoclast-specific markers were significantly downregulated in melatonin-treated BMMs. The melatonin-mediated repression of osteoclast differentiation involved the inhibition of the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signaling pathway. The treatment with SIRT1 inhibitors did not affect osteoclast differentiation but, when supplemented with exogenous hydrogen peroxide, a partial rescue of melatonin-suppressed osteoclastogenesis was observed.
Conclusion
Melatonin at pharmacological doses directly inhibited osteoclastogenesis of BMMs by a ROS-mediated but not a SIRT1-independent pathway.






Similar content being viewed by others
Abbreviations
- BMMs:
-
Bone marrow monocytes
- CCK-8:
-
Cell counting kit-8
- CTRL:
-
Control cells
- CTSK:
-
Cathepsin K
- DCFH2-DA:
-
2′,7′-Dichlorofluorescein diacetate
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- H2O2 :
-
Hydrogen peroxide
- M-CSF:
-
Macrophage-colony stimulating factor
- MLT:
-
Melatonin
- MSCs:
-
Mesenchymal stem cells
- NAM:
-
Nicotinamide
- NF-κB:
-
Nuclear factor κ-light-chain-enhancer of activated B cells
- OC:
-
Osteoclasts
- OCG:
-
Osteoclastogenesis
- OSCAR:
-
Osteoclast-associated receptor
- RANKL:
-
Receptor activator of nuclear factor-κB ligand
- ROS:
-
Reactive oxygen species
- SIRT1:
-
Silent information regulator type 1
- TRAP:
-
Tartrate-resistant acid phosphatase
References
Cappariello A, Maurizi A, Veeriah V, Teti A (2014) The great beauty of the osteoclast. Arch Biochem Biophys 558:70–78
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Karasek M (2004) Melatonin, human aging, and age-related diseases. Exp Gerontol 39:1723–1729
Zhang HM, Zhang Y (2014) Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57:131–146
Maria S, Witt-Enderby PA (2014) Melatonin effects on bone: potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J Pineal Res 56:115–125
Liu X, Gong Y, Xiong K, Ye Y, Xiong Y, Zhuang Z, Luo Y, Jiang Q, He F (2013) Melatonin mediates protective effects on inflammatory response induced by interleukin-1 beta in human mesenchymal stem cells. J Pineal Res 55:14–25
Liu X, Xu Y, Chen S, Tan Z, Xiong K, Li Y, Ye Y, Luo ZP, He F, Gong Y (2014) Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic Biol Med 68:234–246
Amstrup AK, Sikjaer T, Heickendorff L, Mosekilde L, Rejnmark L (2015) Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: a randomized controlled trial. J Pineal Res 59:221–229
Kotlarczyk MP, Lassila HC, O'Neil CK, D'Amico F, Enderby LT, Witt-Enderby PA, Balk JL (2012) Melatonin osteoporosis prevention study (MOPS): a randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J Pineal Res 52:414–426
Detsch R, Boccaccini AR (2015) The role of osteoclasts in bone tissue engineering. J Tissue Eng Regen Med 9:1133–1149
Histing T, Anton C, Scheuer C, Garcia P, Holstein JH, Klein M, Matthys R, Pohlemann T, Menger MD (2012) Melatonin impairs fracture healing by suppressing RANKL-mediated bone remodeling. J Surg Res 173:83–90
Koyama H, Nakade O, Takada Y, Kaku T, Lau KH (2002) Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J Bone Miner Res 17:1219–1229
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33:359–370
Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, Vriend J, Tan DX, Reiter RJ (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 59:403–419
Chen X, Li X, Du Z, Shi W, Yao Y, Wang C, He K, Hao A (2014) Melatonin promotes the acquisition of neural identity through extracellular-signal-regulated kinases 1/2 activation. J Pineal Res 57:168–176
Shakibaei M, Buhrmann C, Mobasheri A (2011) Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem 286:11492–11505
Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan G, Cui W, Luo ZP, Pei M, Yang H, He F (2015) Melatonin reverses H2O2-induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res 59:190–205
Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P (2010) Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J Pineal Res 49:291–300
Zhang L, Su P, Xu C, Chen C, Liang A, Du K, Peng Y, Huang D (2010) Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARgamma expression and enhancing Runx2 expression. J Pineal Res 49:364–372
Sethi S, Radio NM, Kotlarczyk MP, Chen CT, Wei YH, Jockers R, Witt-Enderby PA (2010) Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. J Pineal Res 49:222–238
He F, Liu X, Xiong K, Chen S, Zhou L, Cui W, Pan G, Luo ZP, Pei M, Gong Y (2014) Extracellular matrix modulates the biological effects of melatonin in mesenchymal stem cells. J Endocrinol 223:167–180
Quinn JM, Horwood NJ, Elliott J, Gillespie MT, Martin TJ (2000) Fibroblastic stromal cells express receptor activator of NF-kappa B ligand and support osteoclast differentiation. J Bone Miner Res 15:1459–1466
Choi EY, Jin JY, Lee JY, Choi JI, Choi IS, Kim SJ (2011) Melatonin inhibits Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-6 in murine macrophages by suppressing NF-kappaB and STAT1 activity. J Pineal Res 50:197–206
Shi D, Xiao X, Wang J, Liu L, Chen W, Fu L, Xie F, Huang W, Deng W (2012) Melatonin suppresses proinflammatory mediators in lipopolysaccharide-stimulated CRL1999 cells via targeting MAPK, NF-kappaB, c/EBPbeta, and p300 signaling. J Pineal Res 53:154–165
Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML, Yen ML (2011) Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res 26:2552–2563
Edwards JR, Perrien DS, Fleming N, Nyman JS, Ono K, Connelly L, Moore MM, Lwin ST, Yull FE, Mundy GR, Elefteriou (2013) Silent information regulator (Sir)T1 inhibits NF-kappaB signaling to maintain normal skeletal remodeling. J Bone Miner Res 28:960–969
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859
Ke K, Sul OJ, Choi EK, Safdar AM, Kim ES, Choi HS (2014) Reactive oxygen species induce the association of SHP-1 with c-Src and the oxidation of both to enhance osteoclast survival. Am J Physiol Endocrinol Metab 307:E61–E70
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 42:28–42
Fischer TW, Kleszczynski K, Hardkop LH, Kruse N, Zillikens D (2013) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res 54:303–312
Kleszczynski K, Zillikens D, Fischer TW (2016) Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (gamma-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J Pineal Res 61:187–197
Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, Lee KW, Kang MI (2010) Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int 87:226–235
Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ (2005) Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 146:728–735
Ke K, Safder MA, Sul OJ, Kim WK, Suh JH, Joe Y, Chung HT, Choi HS (2015) Hemeoxygenase-1 maintains bone mass via attenuating a redox imbalance in osteoclast. Mol Cell Endocrinol 409:11–20
Xu Y, Morse LR, da Silva RA, Odgren PR, Sasaki H, Stashenko P, Battaglino RA (2010) PAMM: a redox regulatory protein that modulates osteoclast differentiation. Antioxid Redox Signal 13:27–37
Acknowledgments
The authors are grateful to Suzanne Danley (West Virginia University, USA) and Paula Sahyoun (University of Waterloo, Canada) for carefully reviewing and editing the manuscript. This work was supported by the National Natural Science Foundation of China (31570978, 21574091, 31400826), the Natural Science Foundation of Jiangsu Province (BK20140323), the National Institutes of Health (NIH) (AR062763-01A1, AR067747-01A1) and an Established Investigator Grant from Musculoskeletal Transplant Foundation (MTF) to M.P., and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
ESM 1
(DOCX 794 kb)
Rights and permissions
About this article
Cite this article
Zhou, L., Chen, X., Yan, J. et al. Melatonin at pharmacological concentrations suppresses osteoclastogenesis via the attenuation of intracellular ROS. Osteoporos Int 28, 3325–3337 (2017). https://doi.org/10.1007/s00198-017-4127-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4127-8