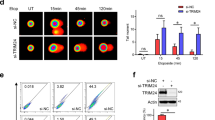
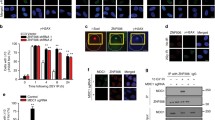
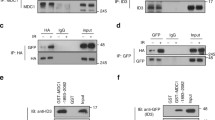
Abstract
Genomic instability is one of the representative causes in genetic disorder, where the proper cellular response to DNA damage is essential in maintaining genomic stability. ATM and the Mre11-Rad50-Nbs1 (MRN) complex play critical roles in the cellular response to DNA damage such as DNA double-strand break (DSB). In this study, we report that Smad7 is indispensible in DNA damage response as a novel component of MRN complex. Smad7 enhances cell survival against DNA damage by accelerating ATM dependent DNA repair signaling. In Smad7-deficient mouse embryonic fibroblast cells, the loss of Smad7 decreases ATM activation and inhibits recruitment of ATM to the sites of DSBs. Smad7 interacts with Nbs1, a member of MRN complex, and enhances the interaction between ATM and Nbs1 upon DNA damage response, leading to phosphorylation of downstream substrates. Ectopic expression of Smad7 in the skin of mice enhances the phosphorylation of ATM upon X-irradiation. We found that effect of Smad7 on enhancing DNA repair is independent of its inhibitory activity of TGF-β signaling. Taken together, our results highlight a critical function of Smad7 in DSB response and establish the novel mechanism in which Smad7 facilitates the recruitment of ATM to the MRN complex through direct interaction with Nbs1.







Similar content being viewed by others
References
Bartek J, Bartkova J, Lukas J (2007) DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 26(56):7773–7779. doi:10.1038/sj.onc.1210881
Negrini S, Gorgoulis VG, Halazonetis TD (2010) Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol 11(3):220–228. doi:10.1038/nrm2858
Jackson SP (2002) Sensing and repairing DNA double-strand breaks. Carcinogenesis 23(5):687–696
Kastan MB, Bartek J (2004) Cell-cycle checkpoints and cancer. Nature 432(7015):316–323. doi:10.1038/nature03097
Lavin MF (2008) Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 9(10):759–769. doi:10.1038/nrm2514
Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y (2003) Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 22(20):5612–5621. doi:10.1093/emboj/cdg541
Paull TT, Lee JH (2005) The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell Cycle 4(6):737–740
Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE (2000) Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res 60(21):5934–5936
Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421(6922):499–506. doi:10.1038/nature01368
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276(45):42462–42467. doi:10.1074/jbc.C100466200
Derheimer FA, Kastan MB (2010) Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett 584(17):3675–3681. doi:10.1016/j.febslet.2010.05.031
Kozlov SV, Graham ME, Peng C, Chen P, Robinson PJ, Lavin MF (2006) Involvement of novel autophosphorylation sites in ATM activation. EMBO J 25(15):3504–3514. doi:10.1038/sj.emboj.7601231
Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, Pelletier L, Jackson SP, Durocher D (2007) Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318(5856):1637–1640. doi:10.1126/science.1150034
Itoh S, Landstrom M, Hermansson A, Itoh F, Heldin CH, Heldin NE, ten Dijke P (1998) Transforming growth factor beta1 induces nuclear export of inhibitory Smad7. J Biol Chem 273(44):29195–29201
Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG (2007) Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol 27(12):4488–4499. doi:10.1128/MCB.01636-06
Zhang S, Ekman M, Thakur N, Bu S, Davoodpour P, Grimsby S, Tagami S, Heldin CH, Landstrom M (2006) TGFbeta1-induced activation of ATM and p53 mediates apoptosis in a Smad7-dependent manner. Cell Cycle 5(23):2787–2795
Okado T, Terada Y, Tanaka H, Inoshita S, Nakao A, Sasaki S (2002) Smad7 mediates transforming growth factor-beta-induced apoptosis in mesangial cells. Kidney Int 62(4):1178–1186. doi:10.1111/j.1523-1755.2002.kid583.x
Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM (2003) Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 125(1):178–191. doi:10.1016/s0016-5085(03)00666-8
Lan HY (2003) Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14(6):1535–1548. doi:10.1097/01.asn.0000067632.04658.b8
Saika S, Ikeda K, Yamanaka O, Miyamoto T, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Nakajima Y, Kao WW, Flanders KC, Roberts AB (2005) Expression of Smad7 in mouse eyes accelerates healing of corneal tissue after exposure to alkali. Am J Pathol 166(5):1405–1418. doi:10.1016/S0002-9440(10)62358-9
Han G, Li F, Ten Dijke P, Wang XJ (2011) Temporal smad7 transgene induction in mouse epidermis accelerates skin wound healing. Am J Pathol 179(4):1768–1779. doi:10.1016/j.ajpath.2011.06.003
Wang M, Saha J, Hada M, Anderson JA, Pluth JM, O’Neill P, Cucinotta FA (2013) Novel Smad proteins localize to IR-induced double-strand breaks: interplay between TGFbeta and ATM pathways. Nucleic Acids Res 41(2):933–942. doi:10.1093/nar/gks1038
Lee JH, Kang Y, Khare V, Jin ZY, Kang MY, Yoon Y, Hyun JW, Chung MH, Cho SI, Jun JY, Chang IY, You HJ (2010) The p53-inducible gene 3 (PIG3) contributes to early cellular response to DNA damage. Oncogene 29(10):1431–1450. doi:10.1038/onc.2009.438
Noon AT, Shibata A, Rief N, Lobrich M, Stewart GS, Jeggo PA, Goodarzi AA (2010) 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat Cell Biol 12(2):177–184. doi:10.1038/ncb2017
He W, Li AG, Wang D, Han S, Zheng B, Goumans MJ, Ten Dijke P, Wang XJ (2002) Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J 21(11):2580–2590. doi:10.1093/emboj/21.11.2580
Falanga V, Schrayer D, Cha J, Butmarc J, Carson P, Roberts AB, Kim SJ (2004) Full-thickness wounding of the mouse tail as a model for delayed wound healing: accelerated wound closure in Smad3 knock-out mice. Wound Repair Regen 12(3):320–326. doi:10.1111/j.1067-1927.2004.012316.x
Wiegman EM, Blaese MA, Loeffler H, Coppes RP, Rodemann HP (2007) TGFbeta-1 dependent fast stimulation of ATM and p53 phosphorylation following exposure to ionizing radiation does not involve TGFbeta-receptor I signalling. Radiother Oncol 83(3):289–295. doi:10.1016/j.radonc.2007.05.013
Cariveau MJ, Tang X, Cui XL, Xu B (2007) Characterization of an NBS1 C-terminal peptide that can inhibit ataxia telangiectasia mutated (ATM)-mediated DNA damage responses and enhance radiosensitivity. Mol Pharmacol 72(2):320–326. doi:10.1124/mol.107.036681
You Z, Chahwan C, Bailis J, Hunter T, Russell P (2005) ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol 25(13):5363–5379. doi:10.1128/MCB.25.13.5363-5379.2005
Perry J, Kleckner N (2003) The ATRs, ATMs, and TORs are giant HEAT repeat proteins. Cell 112(2):151–155
Han G, Bian L, Li F, Cotrim A, Wang D, Lu J, Deng Y, Bird G, Sowers A, Mitchell JB, Gutkind JS, Zhao R, Raben D, ten Dijke P, Refaeli Y, Zhang Q, Wang XJ (2013) Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis. Nat Med 19(4):421–428. doi:10.1038/nm.3118
Hong S, Lee C, Kim SJ (2007) Smad7 sensitizes tumor necrosis factor induced apoptosis through the inhibition of antiapoptotic gene expression by suppressing activation of the nuclear factor-kappaB pathway. Cancer Res 67(19):9577–9583. doi:10.1158/0008-5472.CAN-07-1179
Hong S, Lim S, Li AG, Lee C, Lee YS, Lee EK, Park SH, Wang XJ, Kim SJ (2007) Smad7 binds to the adaptors TAB 2 and TAB 3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat Immunol 8(5):504–513. doi:10.1038/ni1451
Simonsson M, Heldin CH, Ericsson J, Gronroos E (2005) The balance between acetylation and deacetylation controls Smad7 stability. J Biol Chem 280(23):21797–21803. doi:10.1074/jbc.M503134200
Gronroos E, Hellman U, Heldin CH, Ericsson J (2002) Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell 10(3):483–493
Costanzo V, Paull T, Gottesman M, Gautier J (2004) Mre11 assembles linear DNA fragments into DNA damage signaling complexes. PLoS Biol 2(5):E110. doi:10.1371/journal.pbio.0020110
Lee JH, Paull TT (2005) ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308(5721):551–554. doi:10.1126/science.1108297
Lloyd J, Chapman JR, Clapperton JA, Haire LF, Hartsuiker E, Li J, Carr AM, Jackson SP, Smerdon SJ (2009) A supramodular FHA/BRCT-repeat architecture mediates Nbs1 adaptor function in response to DNA damage. Cell 139(1):100–111. doi:10.1016/j.cell.2009.07.043
Lee JH, Goodarzi AA, Jeggo PA, Paull TT (2010) 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J 29(3):574–585. doi:10.1038/emboj.2009.372
Wu J, Zhang X, Zhang L, Wu CY, Rezaeian AH, Chan CH, Li JM, Wang J, Gao Y, Han F, Jeong YS, Yuan X, Khanna KK, Jin J, Zeng YX, Lin HK (2012) Skp2 E3 ligase integrates ATM activation and homologous recombination repair by ubiquitinating NBS1. Mol Cell 46(3):351–361. doi:10.1016/j.molcel.2012.02.018
Kirshner J, Jobling MF, Pajares MJ, Ravani SA, Glick AB, Lavin MJ, Koslov S, Shiloh Y, Barcellos-Hoff MH (2006) Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res 66(22):10861–10869. doi:10.1158/0008-5472.CAN-06-2565
Dubrovska A, Kanamoto T, Lomnytska M, Heldin CH, Volodko N, Souchelnytskyi S (2005) TGFbeta1/Smad3 counteracts BRCA1-dependent repair of DNA damage. Oncogene 24(14):2289–2297. doi:10.1038/sj.onc.1208443
Kanamoto T, Hellman U, Heldin CH, Souchelnytskyi S (2002) Functional proteomics of transforming growth factor-beta1-stimulated Mv1Lu epithelial cells: Rad51 as a target of TGFbeta1-dependent regulation of DNA repair. EMBO J 21(5):1219–1230. doi:10.1093/emboj/21.5.1219
Chang J, Park K, Bang YJ, Kim WS, Kim D, Kim SJ (1997) Expression of transforming growth factor beta type II receptor reduces tumorigenicity in human gastric cancer cells. Cancer Res 57(14):2856–2859
Han G, Li AG, Liang YY, Owens P, He W, Lu S, Yoshimatsu Y, Wang D, Ten Dijke P, Lin X, Wang XJ (2006) Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell 11(3):301–312. doi:10.1016/j.devcel.2006.06.014
Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, Samuni AM, Felici A, Reiss M, Yarkoni S, Sowers A, Mitchell JB, Roberts AB, Russo A (2004) Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem 279(15):15167–15176. doi:10.1074/jbc.M309798200
Monteleone G, Del Vecchio Blanco G, Palmieri G, Vavassori P, Monteleone I, Colantoni A, Battista S, Spagnoli LG, Romano M, Borrelli M, MacDonald TT, Pallone F (2004) Induction and regulation of Smad7 in the gastric mucosa of patients with Helicobacter pylori infection. Gastroenterology 126(3):674–682
Acknowledgments
The authors would like to thank Wang, XJ for the K7.Smad7 transgenic mice and Yoon, K, Choi, CY, and Kim, TK for technical support. This work was supported by Basic Science Research Program (NRF 2011-0014281) and the Bio-Synergy Research Project (NRF-2012M3A9C4048735) of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF).
Conflict of interest
All authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, S., Kang, J.M., Kim, S.J. et al. Smad7 enhances ATM activity by facilitating the interaction between ATM and Mre11-Rad50-Nbs1 complex in DNA double-strand break repair. Cell. Mol. Life Sci. 72, 583–596 (2015). https://doi.org/10.1007/s00018-014-1687-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1687-z