Abstract
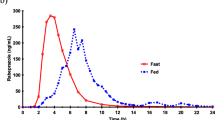
Rectal absorption of phenytoin and its sodium salt from various dosage forms was studied in man. The rectal dosage forms included fatty suppositories, an aqueous suspension and solutions with various solvents in order to achieve complete dissolution of phenytoin. The plasma concentration of phenytoin was measured by means ofHplc analysis after a single dose of 200 mg phenytoin in a cross-over study in eight volunteers. A comparison was made with oral administration. Compared with oral administration rectal absorption conditions of phenytoin from fatty suppositories or aqueous suspensions were found to be extremely unfavourable. Although the addition of alkali and glycofurol increased the rectal absorption, absorption only occurred with an appreciable rate during the first 30 minutes after administration. This in contrast to a rectal solution using polyethylene glycol 600 as a solvent which did produce a slow but continuous absorption over at least 8 hours. Relative bioavailability after this period of time was calculated to be 50%. We conclude that it is in principle possible to improve the rectal absorption rate and also the bioavailability of phenytoin by increasing the solubility of this drug with non-absorbable agents.
Similar content being viewed by others
References
Chadwick, V.S., S.F. Phillips andA.F. Hofmann (1977)Gastroenterology 73, 241–251.
Crommelin, D.J.A., J. Modderkolk andC.J. De Blaey (1979)Int. J. Pharm. 3, 299–309.
Flines, E.W. De (1979)Pharm. Weekblad 114, 805.
Jung, D., J.R. Powell, Ph. Walson andD. Perrier (1980)Clin. Pharmacol. Therap. 28, 479–486.
Lerk, C.F., M. Lagas, L. Lie-A-Huen, P. Broersma andK. Zuurman (1979)J. Pharm. Sci. 68, 634–638.
Meijer, J.W.A., andR. Kalff (1975) In:Clinical pharmacology of anti-epileptic drugs. (Schneider, H., D. Janz, C. Gardner-Thorpe, H. Meinardi andA.L. Sherwin, Eds.). Springler-Verlag, Berlin-Heidelberg-New York, 222–228.
Moolenaar, F. (1979)Biopharmaceutics of rectal administration of drugs in man. Ph.D. thesis, University of Groningen, The Netherlands.
Moolenaar, F., B. Koning andT. Huizinga (1979a)Int. J. Pharm. 4, 99–109.
Moolenaar, F., L. Olthof andT. Huizinga (1979b)Pharm. Weekblad 114, 201–206.
Moolenaar, F., W.J. Greving andT. Huizinga (1980a)Eur. J. Clin. Pharmacol. 17, 309–315.
Moolenaar, F., S. Bakker, J. Visser andT. Huizinga (1980b)Int. J. Pharm. 5, 127–137.
Schobben, F.A.M. (1979)Pharmacokinetics and Therapeutics in epilepsy. Ph.D. thesis, University of Nijmegen, The Netherlands.
Schoonen, A.J.M., F. Moolenaar, C. Haverschmidt andT. Huizinga (1976)Pharm. Weekblad 111, 585–590.
Schoonen, A.J.M., F. Moolenaar andT. Huizinga (1979)Int. J. Pharm. 4, 141–152.
Westenberg, H.G.M., andR.A. De Zeeuw (1976)J. Chrom. 118, 217–224.
Author information
Authors and Affiliations
Additional information
In honour of ProfessorHuizinga on the occasion of his retirement.
Rights and permissions
About this article
Cite this article
Moolenaar, F., Jelsma, R.B.H., Visser, J. et al. Manipulation of rectal absorption rate of phenytoin in man. Pharmaceutisch Weekblad Scientific Edition 3, 1051–1056 (1981). https://doi.org/10.1007/BF02193322
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02193322