Summary
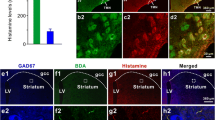
The synaptic connections between histaminergic neurons and substance P (SP) afferents in the caudal magnocellular nucleus (CM) of the hypothalamus were examined using an immunoelectron microscopic mirror method. SP-immunoreactive (SP-IR) terminals made synaptic contacts with the somata, somatic spines and dendrites of histidine decarboxylase immunoreactive (HDC-IR) neurons. This suggests that SP afferents exert monosynaptic influence on the central histaminergic neuronal system.
Similar content being viewed by others
References
Brezenoff HE, Lomax P (1970) Temperature changes following microinjection of histamine into the thermoregulatory centers of the rat. Experientia 26:51–52
Clark WG, Cumby HR (1970) Biphasic changes in body temperature produced by intracerebroventricular injections of histamine in the cat. J Physiol (Lond) 261:235–253
Clineschmidt BV, Lotti VJ (1973) Histamine: intraventricular injection suppresses ingestive behavior of the cat. Arch Int Pharmacodyn Ther 206:288–298
Donoso AO (1986) The possible role of brain histamine in neuroendocrine and cardiovascular regulation. Med Res Rev 6:365–386
Eccles JC (1964) The physiology of synapses. Springer, Berlin Heidelberg New York
Ericson H, Watanabe T, Köhler C (1987) Morphological analysis of the tuberomammillary nucleus in the rat brain: delineation of subgroups with antibody against L-histidine decarboxylase as a marker. J Comp Neurol 263:1–24
Hayashi H, Takagi H, Takeda N, Kubota Y, Tohyama M, Watanabe T, Wada H (1984) Fine structure of histaminergic neurons in the caudal magnocellular nucleus of the rat as demonstrated by immunocytochemistry using histidine decarboxylase as a marker. J Comp Neurol 229:233–241
Hsu S, Raine L, Fanger H (1981) Use of avidin-biotinperoxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Inagaki S, Sakanaka M, Shiosaka S, Senba E, Takatsuji K, Takagi H, Kawai Y, Minagawa H, Tohyama M (1982) Ontogeny of substance-P containing neuron system of the rat: immunohistochemical analysis. I. Forebrain and upper brain stem. Neuroscience 7:251–277
Inagaki N, Yamatodani A, Ando-Yamamoto M, Tohyama M, Watanabe T, Wada H (1988) Organization of histaminergic fibers in the rat brain. J Comp Neurol 273:283–300
Katayama-Kumoi Y, Kiyama H, Manabe R, Shiotani Y, Tohyama M (1985) Coexistence of glucagon- and substance P-like immunoreactivity in the chicken retina. Neuroscience 16:417–424
Kishino J, Takemura M, Mochizuki T, Yamatodani A, Wada H (1989) Regulation of the endogenous histamine release from rat hypothalamic slices. Abstract. Neurosci Res [Suppl] 8 (in press)
Köhler C, Swanson LW, Haglund L, Wu JY (1985) The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience 16:85–110
Ljungdahl Å, Hökfelt T, Nilsson G (1978) Distribution of substance P-like immunoreactivity in the central nervous system of the rat. I. Cell bodies and nerve terminals. Neuroscience 3:861–943
Schwartz JC, Garbarg M, Pollard H (1986) Histaminergic transmission in the brain. In: Mountcastle VB, Bloom FE, Geiger SR (eds) Handbook of physiology, Vol 4. The nervous system. American Physiological Society, Bethesda Md, pp 257–316
Simerly RB, Gorski RA, Swanson LW (1986) Neurotransmitter specificity of cells and fibers in the medial preoptic nucleus: an immunohistochemical study in the rat. J Comp Neurol 246:343–363
Somogyi P, Takagi H (1982) A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry. Neuroscience 7:1779–1783
Steinbusch HWM, Mulder AH (1984) Immunohistochemical localization of histamine in neurons and mast cells in the rat brain. In: Björklund A, Hökfelt T, Kuhar MJ (eds) Handbook of chemical neuroanatomy, Vol 3. Classical transmitters and transmitter receptors in the CNS. Elsevier, Amsterdam, pp 126–140
Steinbusch HWM, Sauren Y, Groenewegen H, Watanabe T, Mulder AH (1986) Histaminergic projections from the premammillary and posterior hypothalamic region to the caudate putamen complex in the rat. Brain Res 368:389–393
Takagi H (1987) Immunoelectron microscopic mirror method: a correlative study. In: Hayat MA (eds) Correlative microscopy in biology: instrumentation and methods. Academic Press, New York, pp 355–366
Tamiya R, Hanada M, Narita N, Kawai Y, Tohyama M, Takagi H (1989) Neuropeptide Y afferents have synaptic interactions with histaminergic (histidine decarboxylase-immunoreactive) neurons in the rat brain. Neurosci Lett 99:241–245
Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, Terano Y, Tohyama M, Wada H (1984) Distribution of the histaminergic neuron system in the central nervous system of rats: a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res 295:13–25
Wouterlood FG, Gaykema RPA (1988) Innervation of histaminergic neurons in the posterior hypothalamic region by medial preoptic neurons: anterograde tracing with phaseolus vulgaris leucoagglutinin combined with immunocytochemistry of histidine decarboxylase in the rat. Brain Res 455:170–176
Wouterlood FG, Sauren YMHF, Steinbusch HWM (1986) Histaminergic neurons in the rat brain: correlative immunocytochemistry, Golgi impregnation, and electron microscopy. J Comp Neurol 252:227–244
Wouterlood FG, Steinbusch HWM, Luiten PGM, Bol JGJM (1987) Projection from the prefrontal cortex to histaminergic cell groups in the posterior hypothalamic region of the rat: anterograde tracing with Phaseolus vulgaris leucoagglutinin combined with immunocytochemistry of histidine decarboxylase. Brain Res 406:330–336
Wouterlood FG, Gaykema RPA, Steinbusch HWM, Watanabe T, Wada H (1988) The connections between the septumdiagonal band complex and histaminergic neurons in the posterior hypothalamus of the rat: anterograde tracing with Phaseolus vulgaris leucoagglutinin combined with immunocytochemistry of histidine decarboxylase. Neuroscience 26:827–845
Zamboni L, De Martino C (1967) Buffered picric acid formaldehydes: a new fixative for electron-microscopy. J Cell Biol 35:148/A
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tamiya, R., Hanada, M., Narita, N. et al. Histaminergic neurons receive substance P-ergic inputs in the posterior hypothalamus of the rat. Exp Brain Res 79, 261–265 (1990). https://doi.org/10.1007/BF00608234
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00608234