Abstract
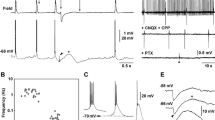
Transient changes in extracellular potassium concentration ([K+]0) and field potentials were evoked by 4-aminopyridine (4-AP; 50–100 μM) and recorded with ion-selective microelectrodes in CA1b, CA3b and dentate sectors of adult rat hippocampal slices. Long-lasting field potentials recurred at a frequency of ≈1/60 s (0.016±0.003 Hz) in association with increases in [K+]0 which were largest and most sustained in the dendritic regions where afferent fibers terminate (dentate>CAl>CA3) and in the hilus. In stratum radiatum of CA1 or stratum moleculare of the dentate these fields had a peak amplitude of 1.4±0.29 mV, duration 8.3±1.6 s, and were accompanied by increases in [K+]0 of 1.8±0.22 mM that lasted 32±5.5 s (n = 17 slices). Interictal epileptiform potentials, which were brief (<0.2 s) and more frequent at ≈1/3 s (0.30±0.02 Hz) were also present in CA1, CA3 and the hilus and associated with small increases in [K+]0 (≤0.5 mM, duration ≤2 s). Interictal activity was blocked by 6-cyano-7-nitroquinoxalone-2,3-dione (CNQX; 5–20 μM); the slow, less frequent potentials were resistant to both CNQX and dl-2amino-5-phosphonovaleric acid (APV; 50 μM) and reversibly blocked (or attenuated by ≈80%) by bicuculline methiodide (BMI) (25–100 μM). The BMI-sensitive potentials were also abolished by baclofen (100 μM), an effect which was reversed by 2-OH-saclofen (100 μM). Focal application of KCl or GABA in the absence of 4-AP evoked long-lasting field and [K+]0 potentials which were similar to those evoked by 4-AP but more sustained. The proportional relationship between the amplitudes of field and K+ potentials with GABA closely resembled that observed for 4-AP; in contrast the slope of KCl-evoked responses was lower. Our results demonstrate that in the adult rat hippocampus 4-AP induces in many different regions accumulations of [K+]0 in synchrony with the long-lasting field potentials, which are known to correspond to an intracellular long-lasting depolarization of the pyramidal cells. These changes are smaller than those which occur in the immature rat hippocampus — which may be related to differences in Na-K-ATPase and susceptibility to seizures. These events involve the activation of GABAA receptors, are under the modulatory control of GABAB receptors, and likely arise from the activity of GABAergic interneurons and/or afferent terminals. The long-lasting field potentials appear to reflect mainly the direct depolarizing actions of GABA and to a much more limited extent the associated accumulation of [K+]0.
Similar content being viewed by others
References
Alger BE, Nicoll RA (1982) Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol (Lond) 328:125–141
Amaral DG (1978) A golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol 182:851–914
Avoli M (1988) GABAergic mechanisms and epileptic discharges. In: Avoli M, Reader TA, Dykes RW, Gloor P (eds) Neurotransmitters and cortical function. Plenum Press, New York, pp 187–205
Avoli M (1992) Synaptic activation of GABAA receptors causes a depolarizing potential under physiological conditions in rat hippocampal pyramidal cells. Eur J Neurosci 4:16–26
Avoli M, Psarrapoulou C, Tancredi V, Fueta Y (1993) On the synchronous activity induced by 4-aminopyridine in the CA3 subfield of juvenile rat hippocampus. J Neurophysiol 70:1018–1029
Barolet AW, Morris ME (1991) Changes in extracellular K+ evoked by GABA, THIP and baclofen in the guinea-pig hippocampal slice. Exp Brain Res 84:591–598
Barolet AW, Kish SJ, Morris ME (1985) Identification of extrasynaptic binding sites for [3H]GABA in peripheral nerve. Brain Res 358:104–109
Barron DH, Matthews BHC (1938) The interpretation of potential changes in the spinal cord. J Physiol (Lond) 92:276–321
Benninger C, Kadis J, Prince DA (1980) Extracellular calcium and potassium changes in hippocampal slices. Brain Res 187:165–182
Buhl EH, Halasy K, Somogyi P (1994) Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368:823–828
Dufau E, Acker H, Sylvester D (1982) Triple-barrelled ion-sensitive microelectrode for simultaneous measurements of two extracellular ion activities. Med Progr Technol 9:33–40
Fisher RS, Pedley TA, Moody WJ, Prince DA (1976) The role of extracellular potassium in hippocampal epilepsy. Arch Neurol 33:76–83
Galvan M, Grafe P, ten Bruggencate G (1982) Convulsant actions of 4-aminopyridine on the guinea-pig olfactory slice. Brain Res 241:75–86
Green ID (1964) The hippocampus. Physiol Rev 44:561–608
Guilbault GG, Durst RA, Frant MS, Freiser H, Hansen EH, Light TS, Pungor E, Rechnitz G, Rice NM, Rohm TJ, Simon W, Thomas JDR (1976) Recommendations for nomenclature of ion-selective electrodes. Pure Appl Chem 48:127–145
Heinemann U, Lux HD, Gutnick MJ (1977) Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Exp Brain Res 27:237–243
Inoue M, Oomura Y, Yakushiji T, Akaike N (1986) Intracellular calcium ions decrease the affinity of the GABA receptor. Nature 324:156–158
Ives AE, Jefferys JGR (1990) Synchronization of epileptiform bursts induced by 4-aminopyridine in the in vitro hippocampal slice preparation. Neurosci Lett: 112:239–245
Jensen MS, Yaari Y (1988) The relationship between interictal and ictal paroxysms in an in vitro model of focal hippocampal epilepsy. Ann Neurol 24:591–598
Kriz N, Sykova E, Ujec E, Vyklicky L (1974) Changes of extracellular potassium concentration induced by neuronal activity in the spinal cord of the cat. I Physiol (Lond) 238:1–15
Krnjevic K, Morris ME (1975) Correlation between extracellular focal potentials and K+ potentials evoked by primary afferent activity. Can J Physiol Pharmacol 53:912–922
Krnjevic K, Morris ME (1981) Electrical and functional correlates of changes in transmembrane ionic gradients produced by neural activity in the central nervous system. In: Zeuthen T (ed) Application of ion-selective microelectrodes. Elsevier, Amsterdam, pp 195–216
Krnjevic K, Morris ME, Reiffenstein RJ (1982a) Stimulation-evoked changes in extracellular K+ and Ca2+ in pyramidal layers of the rat's hippocampus. Can J Physiol Pharmacol 60:1643–1657
Krnjevic K, Morris ME, Reiffenstein RI, Ropert N (1982b) Depth distribution and mechanism of changes in extracellular K+ and Ca2+ concentrations in the hippocampus. Can I Physiol Pharmacol 60:1658–1671
Lacaille J-C, Schwartzkroin PA (1988) Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. I Neurosci 8:1400–1410
Lacaille J-C, Mueller AL, Kunkel DD, Schwartzkroin PA (1987) Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. I Neurosci 7:1979–1993
Louvel J, Avoli M, Kurcewicz I, Pumain R (1994) Extracellular free potassium during synchronous activity induced by 4-aminopyridine in the juvenile rat hippocampus. Neurosci Lett 167:97–100
Lux HD (1974) The kinetics of extracellular potassium: relation to epileptogenesis. Epilepsia 15:375–393
Mac Vicar BA, Tse FW, Crichton SA, Kettenman H (1989) GABA-activated Cl- channels in astrocytes of hippocampal slices. I Neurosci 9:3577–3583
McCarren M, Alger BE (1985) Use-dependent depression of IP-SPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol 53:557–571
Michelson HB, Wong RKS (1991) Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science 253:1420–1423
Miles R, Wong RKS (1987) Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature 329:724–726
Misgeld U, Frotscher M (1986) Postsynaptic-GABAergic inhibition of non-pyramidal neurons in the guinea-pig hippocampus. Neuroscience 19:193–206
Moody WJ, Futamachi KJ, Prince DA (1974) Extracellular potassium activity during epileptogenesis. Exp Neurol 42:248–263
Morris ME, DiCostanzo GA, Barolet A, Sheridan PJ (1983) Role of K+ in GABA (gamma-aminobutyric acid)-evoked depolarization of peripheral nerve. Brain Res 278:127–135
Morris ME, Krnjevic K, MacDonald JF (1985) Changes in intracellular free Ca ion concentration evoked by electrical activity in cat spinal neurons in situ. Neuroscience 14:563–580
Morris ME, Leblond J, Agopyan N, Krnjevic K (1991) Temperature dependence of extracellular ionic changes evoked by anoxia in hippocampal slices. J Neurophysiol 65:157–167
Morris ME, Obrocea GV, Avoli M (1993a) Changes in [K+] and synchronous GABA-mediated potentials revealed by 4-aminopyridine in rat hippocampus. Can J Physiol Pharmacol 71:Axiv-Axv
Morris ME, Obrocea GV, Avoli M (1993b) Role of K+ in GABA (γ-aminobutyric acid)-mediated synchronous potentials evoked in rat hippocampus by 4-AP (4-aminopyridine). Soc Neurosci Abstr 19:1668
Müller W, Misgeld U, Lux HD (1989) γ-Aminobutyric acid-induced ion movements in the guinea pig hippocampal slice. Brain Res 484:184–191
Nicholson C, ten Bruggencate G, Senekowitsch R (1976) Large potassium signals and slow potentials evoked during aminopyridine or barium superfusion in cat cerebellum. Brain Res 113:606–610
Obrocea GV, Morris ME (1993) Comparison of changes evoked by anoxia and GABA (γ-aminobutyric acid) in [K+]0 and [Cl-]0 in the guinea pig hippocampus. Can J Physiol Pharmacol 71:Axviii
Oehme M, Simon W (1976) Microelectrode for potassium ions based on a neutral carrier and comparison of its characteristics with a cation exchanger sensor. Anal Chim Acta 86:21–25
Perreault P, Avoli M (1989) Epileptiform bursts induced by 4-aminopyridine (4AP) in the rat hippocampus: possible mechanisms. Soc Neurosci Abstr 15:1213
Perreault P, Avoli M (1991) Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol 65:771–775
Perreault P, Avoli M (1992) 4-Aminopyridine induced epilepti-form activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci 12:104–115
Prince DA (1978) Neurophysiology of epilepsy. Annu Rev Neurosci 1:395–415
Ribak CE, Harris AB, Vaughn JE, Roberts E (1979) Inhibitory GABAergic nerve terminals decrease at sites of focal epilepsy. Science 205:211–214
Rutecki PA, Lebeda FJ, Johnston D (1987) 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol 57:1911–1924
Segal M (1987) Repetitive inhibitory postsynaptic potentials evoked by 4-aminopyridine in hippocampal neurons in vitro. Brain Res 414:117–130
Segal M, Gutnick MJ (1980) Effects of serotonin on extracellular potassium concentration in the rat hippocampal slice. Brain Res 195:389–401
Siniscalchi A, Avoli M (1992) Modulation by GABAB receptors of spontaneous synchronous activities induced by 4-aminopyridine in the rat hippocampus. Neurosci Lett 148:159–163
Somjen GG (1979) Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol 41:159–177
Sypert GW, Ward AA (1974) Changes in extracellular potassium activity during neocortical propagated seizures. Exp Neurol 45:19–41
Szente M, Pongracz F (1981) Comparative study of aminopyridine-induced seizure activities in primary and mirror foci of cat's cortex. Electroencephalogr Clin Neurophysiol 52:353–367
Taylor CP (1988) How do seizures begin? Clues from hippocampal slices. Trends Neurosci 11:375–378
Thompson SM, Gähwiler B (1989a) Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in vitro. J Neurophysiol 61:501–511
Thompson SM, Gähwiler B (1989b) Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide and membrane potential on ECl in hippocampal CA3 neurons. J Neurophysiol 61:512–523
Thompson SM, Gähwiler B (1989c) Activity-dependent disinhibition. III. Desensitization and GABAB receptor-mediated presynaptic inhibition in the hippocampus. J Neurophysiol 61:524–552
Thompson SM, Capogna M, Scanziani M (1993) Presynaptic inhibition in the hippocampus. Trends Neurosci 16:222–226
Voskuyl RA, Albus H (1985) Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res 342:54–66
Watts AE, Jefferys JGR (1993) Effects of carbamezipine and baclofen on 4-aminopyridine-induced epileptiform activity in rat hippocampal slices. Br J Pharmacol 108:819–823
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morris, M.E., Obrocea, G.V. & Avoli, M. Extracellular K+ accumulations and synchronous GABA-mediated potentials evoked by 4-aminopyridine in the adult rat hippocampus. Exp Brain Res 109, 71–82 (1996). https://doi.org/10.1007/BF00228628
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228628