Abstract
The purpose of this work was to study the effects of warm (37°C) and cold (4°C) ischemia on different mitochondrial functions in rat brain, liver and kidney.
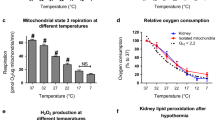
After l0 to 60 minutes of ischemia at 37°C the energy coupled respiration as well as the ADP-induced malate-aspartate shuttle activity in brain and liver mitochondria or the rate of mitochondrial ATP synthesis in kidney were significantly decreased. However, the respiratory rates and the shuttle activity in the absence of ADP remained unchanged. These data suggest that ischemia primarily affects electron transport in the respiratory chain rather than the hydrogen shuttle and the energy coupling system. When the temperature during the indicated ischemic periods was decreased to 4°C, in brain and liver no significant alterations of these mitochondrial functions were found in comparison with the non-ischemic controls. When rat kidneys were stored for 36 hours at 4°C according to Collins mimicing transplantation conditions, the mitochondrial respiration and ATP synthesis were only slightly decreased. It therefore appears that hypothermia can prevent effectively mitochondrial dysfunction due to ischemia.
Similar content being viewed by others
References
Jennings RB, Herdson PB, Sommers HM: Structural and functional abnormalties in mitochondria isolated from ischemic dog myocardium. Lab Invest 20:548–557, 1969
Mergner WJ, Smith MW, Trump BF: Studies on the pathogenesis of ischemic cell injury. XIP/O ratio and acceptor control. Virchows Arch B Cell Path 26:17–26, 1977
Trump BF, Croker BP, Mergner WJ: The role of energy metabolism, ion and water shifts in the pathogenesis of cell injury. In: GW Richter and DG Scarpelli (eds) Cell Membranes: Biological and Pathological Aspects, Williams and Watkins, Baltimore, 1971, p 84
Trump BF, Mergner WJ: Cell injury. In: BW Zwiefach, L Grant and RT McClusky (eds) The Inflammatory Process, Vol. I, 2nd ed. Academic Press, London/New York, 1974, p 115
Baue AE, Sayeed MM: Alterations in the functional capacity of mitochondria in hemorrhagic shock. Surgery 68:40–47, 1970
Boime J, Smith EE, Hunter Jr FE: Stability of oxidative phosphorylation and structural changes of mitochondria in ischemic rat liver. Arch of Biochem and Biophys 128:704–715, 1968
Boime J, Smith EE, Hunter Jr FE: The role of fatty acids in mitochondrial changes during liver ischemia. Arch of Biochem and Biophys 139:425–443, 1970
Daniel AM, Beaudoin J-G: Evaluation of mitochondrial function in the ischemic rat liver. J Surg Res 17:19–25, 1974
Mela L, V. Bacalzo Jr. L, Miller LD: Defective oxiditative metabolism of rat liver mitochondria in hemorrhagic and endotoxin shock. Am J Physiol 220:571–577, 1971
Mittnacht Jr. S, Shermann SC, Farber JL: Reversal of ischemic mitochondrial dysfunction. J Biol Chem 254:9871–9878, 1979
Rhodes RS, DePalma RG, Druet RL: Reversibility of ischemically induced mitochondrial dysfunction with reperfusion. Surg Gynecol Obstet 145:719–724, 1977
Hems DA, Brosnan JT Effects of ischemia on content of metabolites in rat liver and kidney in vivo. Biochem J 120:105–111, 1970
Chien KR, Abrams J, Serroni A, Martin JT, Farber JL: Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem 253:4809–4817, 1978
Chance B, Williams GR: Respiratory enzymes in oxidative phosphorylation: III The steady state. J Biol Chem 217:409–427, 1955
Cooper C, Lehninger AL: Oxidative phosphorylation by an enzyme complex from extracts of mitochondria. J Biol Chem 219:489–506, 1956
Schneider WC: Intracellular distribution of enzymes. J Biol Chem 176:259–276, 1948
Clark JB, Nicklas WJ: The metabolism of rat brain mitochondria. J Biol Chem 245:4724–4731, 1970
Collins GM, Bravo-Shugarman M, Terasaki PI: Kidney preservation for transportation: Initial perfusion and 30 hours' ice storage. Lancet 2:1219–1222, 1969
Berdanier CD, McNamara S: Aging and mitochondrial activity in BHE and Wistar strains of rats. Exp Geront 15:519–525, 1980
Malis ChD, Bonventre JV: Mechanism of Calcium potentiation of oxygen free radical injury to renal mitochondria. J Biol Chem 261:14201–14208, 1986
Southard JH, Senzig KA, Hoffmann RM, Belzer FO: Energy metabolism in kidneys stored by simple hypothermia. Transpl Proc IX 3:1535–1539, 1977
Rouslin W: Protonic Inhibition of the mitochondrial Oligomycin-sensitive Adenosine 5′-triphosphatase in ischemic and autolyzing cardial muscle. J Biol Chem 258:9657–9661, 1983
Rouslin W: Mitochondrial complexes I, II, III, IV and V in myocardial ischemia and autolysis. Am J Physiol: H 743, 1983
Gupte SS, Hackenbrock CR: The role of cytochrome c diffusion in mitochondrial electron transport. J Biol Chem 263:5248–5253, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baumann, M., Bender, E., Sömmer, G. et al. Effects of warm and cold ischemia on mitochondrial functions in brain, liver and kidney. Mol Cell Biochem 87, 137–145 (1989). https://doi.org/10.1007/BF00219256
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00219256