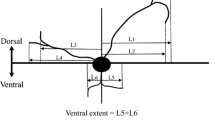
Abstract
Tongue-projecting salamanders (Bolitoglossini) combine extreme speed and high precision in prey capture. They possess all requirements for stereoscopic depth perception: frontally oriented eyes, a substantial amount of direct ipsilateral projection in addition to the contralateral one, and binocularly driven neurons. Extracellular recordings were made from retinal afferents in the tectum as well as from the somata of tectal neurons. RF-sizes of afferents and tectal neurons were determined, and the response properties of tectal neurons were tested under monocular and binocular conditions with stimuli of different size and velocity. While RF-sizes and response properties of binocular neurons during binocular and contralateral stimulation were similar, ipsilaterally stimulated neurons exhibited much smaller RFs, lower spike rates and different size preferences.
Furthermore, the contralateral retinotectal projection from one eye and the ipsilateral from the other are in register. While retinal afferents are distributed linearly over the tectal surface, most tectal neurons are activated by a retinal area corresponding to the frontal visual field; this results in a magnification of this region. The two monocular receptive fields of binocular neurons exhibit zero disparities (horopter) at distances that coincide with the maximum reach of the tongue. We hypothesize that bolitoglossine salamanders (as well as amphibians in general) make use of two kinds of disparities: (1) between the maps in the left and right tectal hemisphere, coding for the lateral eccentricity of an object, and (2) between the ipsilateral and contralateral retinotectal map, coding for the distance. The presence of substantial direct ipsilateral afferents in bolitoglossine salamanders appears to be the basis for a fast computation of object distance, which is characteristic of these animals.
Similar content being viewed by others
Abbreviations
- Ax/Ay :
-
coordinates of a recorded afference
- Nx/Ny :
-
coordinates of a recorded neuron
- RF :
-
receptive field
- RFc :
-
contralateral receptive field
- RFi :
-
ipsilateral receptive field
- RFx/RFy :
-
coordinates of a receptive field center
- RGC :
-
retinal ganglion cell
References
Bousfield JD, Pessoa VF (1980) Changes in ganglion cell density during the post-metamorphotic development in neotropical frog Hyla raniceps. Vision Res 20: 501–510
Collett TS (1977) Stereopsis in toads. Nature 267: 349–351
Douglas RH, Collett TS, Wagner HJ (1986) Accommodation in anuran Amphibia and its role in depth vision. J Comp Physiol A 158: 133–143
Dunlop SA, Beazley LD (1981) Changing retinal ganglion cell distribution in the frog Heleioporus eyrei. J Comp Neurol 202: 221–236
Eurich C, Roth G, Schwegler H, Wiggers W (1995) Simulander: A neural network model for the orientation movement of salamanders. J Comp Physiol A 176: 379–389
Finch DJ, Collett TS (1983) Small-field binocular neurons in the superficial layers of the frog optic tectum. Proc R Soc London B 217: 491–497
Finkenstaedt T, Ewert JP (1983) Processing of area dimensions of visual key stimuli by tectal neurons in Salamandra salamandra. J Comp Physiol 153: 85–98
Fritzsch B (1980) Retinal projections in European Salamandridae. Cell Tissue Res 213: 325–341
Gaillard J (1985) Binocularly driven neurons in the rostral part of the frog optic tectum. J Comp Physiol A 157: 47–55
Gaillard F, Galand G (1977) New ipsilateral visual units in the frog tectum. Brain Res 136: 351–354
Grobstein P (1988) Between the retinotectal projection and directed movement: Topography of a sensorimotor interface. Brain Behav Evol 31: 34–48
Grobstein P, Comer C (1983) The nucleus isthmi as an intertectal relay for the ipsilateral oculotectal projection in the frog, Rana pipiens. J Comp Neurol 217: 54–74
Gruberg ER (1972) Optic fiber projections in the tiger salamander Ambystoma tigrinum. J Hirnforsch 14: 399–411
Gruberg ER, Udin SB (1978) Topographic projections between the nucleus isthmi and the tectum of the frog Rana pipiens. J Comp Neurol 179: 487–500
Jordan M, Luthardt G, Meyer-Naujoks C, Roth G (1980) The role of eye accommodation in the depth perception of common toads. Z Naturforsch 35C: 851–852
Liebmann PA, Entine G (1968) Visual pigments of frog and tadpole (Rana pipiens). Vision Res 8: 761–775
Linke R, Roth G (1988) Neuroglial cells and axons in the optic nerve of lungless salamanders (Fam. Plethodontidae) In: Elsner N, Barth FG (eds) Sense organs. Thieme Stuttgart, p 255
Linke R, Roth G (1990) Optic nerves in plethodontid salamanders (Amphibia, Urodela): neuroglia, fiber spectrum and myelination. Anat Embryol 181: 37–48
Linke R, Roth G, Rottluff B (1986) Comparative studies on the eye morphology in lungless salamanders, family Plethodontidae, and the effect of miniaturization. J Morphol 189: 131–143
Luthardt-Laimer G (1981) Distance estimation in binocular and monocular salamanders. Z Tierpsychol 63: 233–240
Manteuffel G, Fox B, Roth G (1989) Topographic relationships of ipsi- and contralateral visual inputs to the rostral tectum opticum in the salamander Plethodon jordani indicate the presence of a horopter. Neurosci Lett 107: 105–109
Maturana HR (1959) Number of fibers in the optic nerve and the number of ganglion cells in the retina of anurans. Nature 183: 1406–1407
Nguyen VS, Straznicky C (1989) The development and the topographic organization of the retinal ganglion cell layer in Bufo marinus. Exp Brain Res 75: 345–353
Pettigrew JD (1965) A study of binocular interaction in single units of the striate cortex of the cat. Thesis, University of Sidney, Australia
Pettigrew JD (1979) Binocular visual processing in the owl's telencephalon. Proc R Soc Lond B 204: 435–454
Pettigrew JD, Konishi M (1976) Neurons selective for orientation and binocular disparity in the visual wulst of the barn owl (Tyto alba). Science 193: 675–678
Picouet MJ, Clairambault P (1976) Une nouvelle voie visuelle chez un amphibien anoure, Discoglossus pictus. C R Acad Sci (Paris) 282: 16–29
Poggio GF, Fisher B (1977) Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving rhesus monkey. J Neurophysiol 40: 1392–1405
Porciatti V, Fontanesi G, Raffaelli A, Bagnola P (1990) Binocularity in the little owl, Athene noctua. Brain Behav Evol 35: 40–48
Rettig G (1984) Neuroanatomische Untersuchungen der visuellen Projektionen bei Salamandern (Ordnung Caudata). Thesis, Universität Bremen
Rettig G (1988) Connections of the tectum opticum in two urodeles, Salamandra salamandra and Bolitoglossa subpalmata, with special reference to the nucleus isthmi. J Hirnforsch 29: 5–16
Rettig G, Roth G (1982) Afferent visual projections in three species of lungless salamanders (Family Plethodontidae). Neurosci Lett 31: 221–224
Rettig G, Roth G (1986) Retinofugal projections in salamanders of the family Plethodontidae. Cell Tissue Res 243: 285–296
Rossel S (1986) Binocular spatial localization in the praying mantis. J Exp Biol 120: 265–281
Roth G (1976) Experimental analysis of the prey catching behavior of Hydromantes italicus Dunn (Amphibia, Plethodontidae). J Comp Physiol 109: 47–58
Roth G (1987) Visual behavior in salamanders. Springer Berlin
Roth G, Naujoks-Manteuffel C, Grunwald W (1990) Cytoarchitecture of the tectum mesencephali in salamanders: A Golgi and HRP study. J Comp Neurol 291: 27–42
Roth G, Rottluff B, Grunwald W, Hanken J, Linke R (1990) Miniaturization in plethodontid salamanders (Caudata: Plethodontidae) and its consequences for the brain and visual system. Biol J Linnean Soc 40: 165–190
Straub A (1993) Mathematische Modelle für das visuomotorische Verhalten von Amphibien. Thesis, Universität Bremen
Thexton AJ, Wake DB, Wake MH (1977) Tongue function in the salamander Bolitoglossa occidentalis. Arch Oral Biol 22: 361–366
Werner C (1983) Zielorientierung im Beutefangverhalten des Feuersalamanders Salamandra salamandra L. Thesis, Technische Hochschule Darmstadt
Wiggers W (1991) Elektrophysiologische, neuroanatomische und verhaltensphysiologische Untersuchungen zur visuellen Verhaltenssteuerung bei lungenlosen Salamandern. Thesis, Universität Bremen
Wong ROL (1989) Morphology and distribution of neurons in the retina of the American garter snake Thamnophis sirtalis. J Comp Neurol 283: 587–601
Zhang Y, Straznicky C (1991) The morphology and distribution of photoreceptors in the retina of Bufo marinus. Anat Embryol 183: 97–104
Zhu BS, Hiscock J, Straznicky C (1990) The changing distribution of neurons in the inner nuclear layer from metamorphosis to adult: a morphometric analysis of the anuran retina. Anat Embryol 181: 585–594
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wiggers, W., Roth, G., Eurich, C. et al. Binocular depth perception mechanisms in tongue-projecting salamanders. J Comp Physiol A 176, 365–377 (1995). https://doi.org/10.1007/BF00219062
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00219062