Abstract
Purpose
Cadherin switch, as a key hallmark of epithelial–mesenchymal transition (EMT), is characterized by reduced E-cadherin expression and increased N-cadherin or P-cadherin expression, and has been implicated in many aggressive tumors, but the importance and regulatory mechanism of cadherin switch in cervical cancer have not been investigated. Our study aimed to explore the role of cadherin switch by regulation of HPV-16 E6/E7 in progression and metastasis of cervical cancer.
Methods
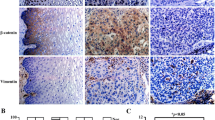
The expressions of E-cadherin and P-cadherin were examined by immunohistochemical staining in 40 cases of high-grade cervical lesions with HPV-16 infection only in which HPV-16 E6 and E7 expression had been detected using qRT-PCR method. Through modulating E6 and E7 expression using HPV-16 E6/E7 promoter-targeting siRNAs or expressed vector in vitro, cell growth, migration, and invasion were separately tested by MTT, wound-healing and transwell invasion assays, as well as the expressions of these cadherins by western blot analyses. Finally, the expressions of these cadherins in cancerous tissues of BALB/c-nu mouse model inoculated with the stable HPV-16 E6/E7 gene silencing Siha and Caski cells were also measured by immunohistochemical staining.
Results
Pearson correlation coefficient analyses showed the strongly inverse correlation of E-cadherin expression and strongly positive correlation of P-cadherin expression with E6/E7 level in 40 cases of high-grade cervical lesions. Furthermore, the modulation of HPV-16 E6/E7 expression remarkably influenced cell proliferation, migration, and invasion, as well as the protein levels of E-cadherin and P-cadherin in cervical cell lines. Finally, the reduction of HPV-16 E6/E7 expression led to up-regulated expression of E-cadherin and down-regulated expression of P-cadherin in BALB/c-nu mouse model in vivo assay.
Conclusions
Our results unraveled the possibility that HPV-16 E6/E7 could promote cell invasive potential via regulating cadherin switching, and consequently contribute to progression and metastasis of cervical cancer.






Similar content being viewed by others
References
Zhai Y, Hotary KB, Nan B et al (2005) Expression of membrane type 1 matrix metalloproteinase is associated with cervical carcinoma progression and invasion. Cancer Res 65:6543–6550
Walboomers JM, Jacobs MV, Manos MM et al (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19
Moody CA, Laimins LA (2010) Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 10:550–560
Boulenouar S, Weyn C, Van Noppen M et al (2010) Effects of HPV-16 E5, E6 and E7 proteins on survival, adhesion, migration and invasion of trophoblastic cells. Carcinogenesis 31:473–480
Spanos WC, Hoover A, Harris GF et al (2008) The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J Virol 82:2493–2500
Jing Y, Han Z, Zhang S et al (2011) Epithelial-Mesenchymal Transition in tumor microenvironment. Cell Biosci 1:29
Iwatsuki M, Mimori K, Yokobori T et al (2010) Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 101:293–299
Thiery JP, Acloque H, Huang RY et al (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Lee MY, Chou CY, Tang MJ et al (2008) Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res 14:4743–4750
Hsu YM, Chen YF, Chou CY et al (2007) KCL cotransporter-3 down-regulates E-cadherin/beta-catenin complex to promote epithelial-mesenchymal transition. Cancer Res 67:11064–11073
Wheelock MJ, Shintani Y, Maeda M et al (2008) Cadherin switching. J Cell Sci 121:727–735
Ye J, Cheng X, Chen X et al (2010) Prevalence and risk profile of cervical Human papillomavirus infection in Zhejiang Province, southeast China: a population-based study. Virol J 7:66
Li BH, Zhou JS, Ye F et al (2011) Reduced miR-100 expression in cervical cancer and precursors and its carcinogenic effect through targeting PLK1 protein. Eur J Cancer 47:2166–2174
Li B, Hu Y, Ye F et al (2010) Reduced miR-34a expression in normal cervical tissues and cervical lesions with high-risk human papillomavirus infection. Int J Gynecol Cancer 20:597–604
Zhou J, Li B, Peng C et al (2013) Inhibition of cervical cancer cell growth in vitro and in vivo by lentiviral-vector mediated shRNA targeting the common promoter of HPV16 E6 and E7 oncogenes. Antiviral Res 98:305–313
Gu J, Liang Y, Qiao L et al (2013) Expression analysis of URI/RMP gene in endometrioid adenocarcinoma by tissue microarray immunohistochemistry. Int J Clin Exp Pathol 6:2396–2403
Lilien J, Balsamo J, Arregui C et al (2002) Turn-off, drop-out: functional state switching of cadherins. Dev Dyn 224:18–29
Paredes J, Figueiredo J, Albergaria A et al (2012) Epithelial E- and P-cadherins: role and clinical significance in cancer. Biochim Biophys Acta 1826:297–311
Bryan RT, Atherfold PA, Yeo Y et al (2008) Cadherin switching dictates the biology of transitional cell carcinoma of the bladder: ex vivo and in vitro studies. J Pathol 215:184–194
Araki K, Shimura T, Suzuki H et al (2011) E/N- cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer 105:1885–1893
Cheung PY, Yip YL, Tsao SW et al (2011) Id-1 induces cell invasiveness in immortalized epithelial cells by regulating cadherin switching and Rho GTPases. J Cell Biochem 112:157–168
Zhang J, Cheng Q, Zhou Y et al (2013) Slug is a key mediator of hypoxia induced cadherin switch in HNSCC: correlations with poor prognosis. Oral Oncol 49:1043–1050
Shah GV, Muralidharan A, Gokulgandhi M et al (2009) Cadherin switching and activation of beta-catenin signaling underlie proinvasive actions of calcitonin-calcitonin receptor axis in prostate cancer. J Biol Chem 284:1018–1030
Lindley LE, Briegel KJ (2010) Molecular characterization of TGFbeta-induced epithelial-mesenchymal transition in normal finite lifespan human mammary epithelial cells. Biochem Biophys Res Commun 399:659–664
Cheung LW, Leung PC, Wong AS (2010) Cadherin switching and activation of p120 catenin signaling are mediators of gonadotropin-releasing hormone to promote tumor cell migration and invasion in ovarian cancer. Oncogene 29:2427–2440
Hao L, Ha JR, Kuzel P et al (2012) Cadherin switch from E- to N-cadherin in melanoma progression is regulated by the PI3 K/PTEN pathway through Twist and Snail. Br J Dermatol 166:1184–1197
Huang J, Xiao D, Li G et al (2014) EphA2 promotes epithelial-mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer cells. Oncogene 33:2737–2747
Watson RA, Thomas M, Banks L et al (2003) Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J Cell Sci 116:4925–4934
Caberg JH, Hubert PM, Begon DY et al (2008) Silencing of E7 oncogene restores functional E-cadherin expression in human papillomavirus 16-transformed keratinocytes. Carcinogenesis 29:1441–1447
Haga T, Uchide N, Tugizov S et al (2008) Role of E-cadherin in the induction of apoptosis of HPV16-positive CaSki cervical cancer cells during multicellular tumor spheroid formation. Apoptosis 13:97–108
Acknowledgments
We thank financial support by grants from the National Nature Science Foundation of China (No. 81202066 and No. 81302248).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Hu and J. Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hu, D., Zhou, J., Wang, F. et al. HPV-16 E6/E7 promotes cell migration and invasion in cervical cancer via regulating cadherin switch in vitro and in vivo. Arch Gynecol Obstet 292, 1345–1354 (2015). https://doi.org/10.1007/s00404-015-3787-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3787-x