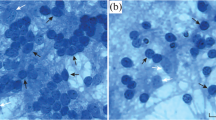
Abstract
We studied effect of gangliosides on viability of brain neurons and neuronal PC12 cell line exposed to toxic concentrations of compounds activating free radical reactions. It is found that preincubation of cerebellar granule cells and PC12 cells with micromolar concentrations of ganglioside GM1 increases statistically significantly viability of these cells submitted to inductors of oxidative stress, such as hydrogen peroxide and the Fe2+-ascorbate system However, the effect of ganglioside GM1 in the PC12 cells failed to be revealed 1–2 days after treatment of the cells with trypsin, which indicates an importance of interaction of gangliosides with surface proteins for realization of their protective action. GM1, GD1a, and other gangliosides were shown to produce the neuroprotective effect on cerebellar granule cells in the presence of toxic glutamate concentrations. Not only micro-, but also nanomolar concentrations of these gangliosides increased statistically significantly the neuronal viability, although at micromolar concentrations this effect as a rule was more pronounced. The obtained data allow suggesting that the neuroprotective action of gangliosides is determined to a considerable degree by their ability to inhibit free-radical reactions in nerve cells.
Similar content being viewed by others
REFERENCES
Mahadik, S.P., Bharucha, V.A., Stadlin, A., Ortiz, A., and Karpiak, S.E., Loss and Recovery of Activities of Alpha+ and Alpha Isozymes of (Na+,K+-ATPase in Cortical Focal Ischemia: GM1 Ganglioside Protects Plasma Membrane Structure and Function, J. Neurosci. Res., 1992, vol. 32, pp. 209–220.
Costa, T., Armstrong, D., Guidotti, A., Kharlamov, A., Kiedrowski, I., and Wroblewski, J.T., Ganglioside GM1 and Its Semisynthetic Lysoganglioside Analogues Reduce Glutamate Neurotoxicity by a Novel Mechanism, Adv. Exp. Biol. Med., 1993, vol. 341, pp. 129–141.
Hadjiconstantinou, M. and Neff, N.H., GM1 Gang lioside: in vivo and in vitro Trophic Actions on Central Neurotransmitter Systems, J. Neurochem., 1998, vol. 70, pp. 1335–1345.
Lazarewicz, J.W., Salinska, E., and Matyja, E., Ganglioside GM1 Prevents N-Methyl-D-Aspartate Neurotoxicity in Rabbit Hippocampus in vivo. Effects on Calcium Homeostasis, Mol. Chem. Neuropathol., 1995, vol. 24, pp. 165–177.
Favaron, M., Manev, H., Alho, H., Bertolino, M., Ferret, B., Guidotti, A., and Costa, E., Gangliosides Prevent Glutamate and Kainate Neurotoxicity in Primary Neuronal Cultures of Neonatal Rat Cerebellum and Cortex, Proc. Natl Acad. Sci. USA, 1988, vol. 85, pp. 7351–7355.
Avrova, N.F., Victorov, I.V., Tyurin, V.A., Zakharova, I.O., Sokolova, T.V., Andreeva, N.A., Stelmaschuk, E.V., Tyurina, Y.Y., and Gonchar, V.S., Inhibition of Glutamate-Induced Intensification of Free Radical Reactions by Gangliosides: Possible Role in Their Protective Effect in Rat Cerebellar Granule Cells and Brain Synaptosomes, Neurochem. Res., 1998, vol. 23, pp. 945–952.
Mattson, M.P., Lovell, M.A., Furukawa, K., and Markesbery, W.R., Neurotrophic Factors Attenuate Glutamate-Induced Accumulation of Peroxides, Elevation of Intracellular Ca2+ Concentration, and Neurotoxicity and Increase Antioxidant Enzyme Activities in Hippocampal Neurons, J. Neurochem., 1995, vol. 65, pp. 1740–1751.
Wilkin, G.P., Balazs, R., Wilson, J.E., Cohen, J., and Dutton, G.R., Preparation of Cell Bodies from the Developing Cerebellum: Structural and Metabolic Integrity of the Isolated Cells, Brain Res., 1976, vol. 115, pp. 181–199.
Andreeva, N., Khodorov, B., Stelmashook, E., Cragoe, E., Jr., and Victorov, I., Inhibition of Na+/Ca2+ Exchange Enhances Delayed Neuronal Death Elicited by Glutamate in Cerebellar Granule Cell Cultures, Brain Res., 1991, vol. 548, pp. 322–325.
Victorov, I.V., Method of Total Staining of Monolayer Cultures with Vanadium Hematoxylin, Byul. Eksp. Biol. Med., 1990, vol. 109, pp. 612–613.
Vassault, A., Lactate Dehydrogenase: UV-Method with Pyruvate and NADH, Methods of Enzymatic Analysis, Bergmeyer, H.U., Ed., Weinheim: Verlag Chemie, 1983, vol. 3, pp. 118–126.
Folch-Pi, J., Lees, M., and Sloan-Stanley, G.H., A Simple Method for Isolation and Purification of Total Lipids from Animal Tissue, J. Biol. Chem., 1957, vol. 226, pp. 497–509.
Svennerholm, L., Mansson, J.E., and Li, Y.T., Isolation and Structural Determination of a Novel Ganglioside, a Disialosylpentahexosylceramide from Human Brain, J. Biol. Chem., 1973, vol. 248, pp. 740–742.
Tyurin, V.A., Tyurina, Y.Yu., and Avrova, N.F., Ganglioside-Dependent Factor, Inhibiting Lipid Peroxidation in Rat Brain Synaptosomes, Neurochem. Int., 1992, vol. 20, pp. 401–407.
Avrova, N.F., Zakharova, I.O., Tyurin, V.A., Tyurina, Y.Y., Gamaley, I.A., and Schepetkin, I.A., Different Metabolic Effects of Ganglioside GM1 in Brain Synaptosomes and Phagocytic Cells, Neurochem. Res., 2002, vol. 27, pp. 751–759.
Saqr, H.E., Pearl, D.K., and Yates, A.J., A Review and Predictive Models of Ganglioside Uptake by Biological Membranes, J. Neurochem., 1993, vol. 61, pp. 395–411.
Cole, K.K. and Perez-Polo, J.R., Poly(ADP-ribose) Polymerase Inhibition Prevents Both Apoptotic-Like Delayed Neuronal Death and Necrosis after H2O2 Injury, J. Neurochem., 2002, vol. 82, pp. 19–29.
Ferrari, G., Anderson, B.L., Stephens, R.M., Kaplan, D.R., and Greene, L.A., Prevention of Apoptotic Neuronal Death by GM1 Ganglioside. Involvement of Trk Neurotrophin Receptors, J. Biol. Chem., 1995, vol. 270, pp. 3074s–3080.
Maroto, R. and Perez-Polo, J.R., BCL-2-Related Protein Expression in Apoptosis: Oxidative Stress Versus Serum Deprivation in PC12 Cells, J. Neuro chem., 1997, vol. 69, pp. 514–523.
Saito, Y., Yoshida, Y., Akazawa, T., Takahashi, K., and Niki, E., Cell Death Caused by Selenium Deficiency and Protective Effect of Antioxidants, J. Biol. Chem., 2003, vol. 278, pp. 39 428–39 434.
Schubert, D., Kimura, H., and Maher, P., Growth Factors and Vitamin E Modify Neuronal Glutamate Toxicity, Proc. Natl Acad. Sci. USA, 1992, vol. 89, pp. 8264–8267.
Maulik, N., Avrova, N., Denisova, N., Madhuri, G., Cordis, G., and Das, D.K., Free Radical Scavenging Activities of Gangliosides, a Sialic Acid Containing Glycosphingolpid, Oxygen Radicals. Proc. of the 5th Intern. Congress in Oxygen Radicals: Active Oxygen, Lipid Peroxides and Antioxidants, Yagi, K., Kondo, M., Niki, E., and Yoshikawa, T., Eds., Amsterdam, London, New York, Tokyo, Excerpta Medica, 1992, pp. 765–768.
Bianco, J.D., Fidelio, C.D., and Maggio, B., Effect of Sulfatide and Gangliosides on Phospholipase C and Phospholipase A2 Activity, A Monolayer Study, Biochim. Biophys. Acta, 1990, vol. 1026, pp. 179–185.
Dawson, T.M., Hung, K., Dawson, V.L., Steiner, J.P., and Snyder, S.H., Neuroprotective Effects of Gangliosides May Involve Inhibition of Nitric Oxide Synthase, Ann. Neurol., 1995, vol. 37, pp. 115–118.
Nalivaeva, N.N., Rybakina, E.G., Shanin, S.N., Kozinets, I.A., and Pivanovich, I.Yu., Ganglioside GM1 Potentiates the Effect of IL-1 Beta on Neutral Sphingomyelinase Activity in Rat Brain Synaptosomes, Biochem. Soc. Trans., 1997, vol. 25, p. 214S.
Prinetti, A., Prioni, S., Chigorno, V., Karagogeos, D., Tettamanti, G., and Sonnino, S., Immunoseparation of Sphingolipid-Enriched Membrane Domains Enriched in Src Family Protein Tyrosine Kinases and in the Neuronal Adhesion Molecule TAG-1 by Anti-GD3 Ganglioside Monoclonal Antibody, J. Neurochem., 2001, vol. 78, pp. 1162–1167.
Chigorno, V., Palestini, P., Sciannamblo, M., Dolo, V., Pavan, A., Tettamanti, G., and Sonnino, S., Evidence That Ganglioside Enriched Domains Are Distinct from Caveolae in MDCK II and Human Fibroblast Cells in Culture, Eur. J. Biochem., 2000, vol. 267, pp. 4187–4197.
Author information
Authors and Affiliations
Additional information
__________
Translated from Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, Vol. 41, No. 4, 2005, pp. 332–338.
Original Russian Text Copyright © 2005 by Sokolova, Furaev, Victorov, Andreeva, Avrova.
Rights and permissions
About this article
Cite this article
Sokolova, T.V., Furaev, V.V., Victorov, I.V. et al. Stimulation by Gangliosides of Viability of Rat Brain Neurons and of Neuronal PC12 Cell Line under Conditions of Oxidative Stress. J Evol Biochem Phys 41, 415–423 (2005). https://doi.org/10.1007/s10893-005-0077-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10893-005-0077-4