Summary
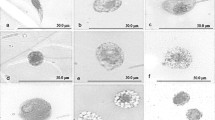
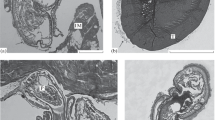
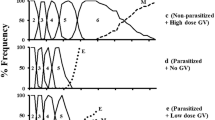
Immature stages of the ichneumonid parasitoid, Campoletis sonorensis, develop within the haemocoel of its noctuid host, Heliothis virescens. The host cannot encapsulate the parasitoid egg owing to the suppressive effect of the polydnavirus-laden calyx fluid injected by the female parasitoid during oviposition. We have examined the effects of injection of calyx fluid on the following haemocytic manifestations of the immune system of 5th-instar larvae of H. virescens: encapsulation, nodulation, phagocytosis, erythrocyte rosetting and coagulation. Of these phenomena, only those requiring the formation of a multicellular sheath of plasmatocytes were affected. In general, encapsulation was fully suppressed; all of the C. sonorensis eggs and most of the glass rods implanted as targets were devoid of attached haemocytes 3 days after implantation although a few of the latter were coated by a sparsely distributed layer of granulocytes. Plasmatocytes also appeared to be present in thicker depositions of haemocytes. In nodulation, only the second, encapsulation-like phase was inhibited. The resistant first stage, involving the entrapment of particles by haemocytes, only resulted in the formation of amorphous, disorganized nodules. Granulocyte-dependent aspects of the immune system (phagocytosis, rosetting and possibly coagulation and the first stage of encapsulation and nodulation) occurred normally. The data suggest that in 5th-instar hosts injection of calyx fluid acts specifically on plasmatocyte function.
Similar content being viewed by others
References
Armstrong PB, Levin J (1979) In vitro phagocytosis by Limulus blood cells. J Invertebr Pathol 34:145–151
Blissard GW, Vinson SB, Summers MD (1986) Identification, mapping, and in vitro translation of Campoletis sonorensis virus mRNAs from parasitized Heliothis virescens larvae. J Virol 57:318–327
Cook DI, Stoltz DB, Vinson SB (1984) Induction of a new haemolymph glycoprotein in larvae of permissive hosts parasitized by Campoletis sonorensis. Insect Biochem 14:45–50
Davies DH, Preston TM (1985) The behaviour of insect plasmatocytes in vitro: changes in morphology during settling, spreading, and locomotion. J Exp Zool 236:71–82
Davies DH, Vinson SB (1986) Passive evasion by eggs of braconid parasitoid Cardiochiles nigriceps of encapsulation in vitro by haemocytes of host Heliothis virescens. Possible role for fibrous layer in immunity. J Ins Physiol 32:1003–1010
Davies DH, Strand MR, Vinson SB (1987) Changes in differential haemocyte count and in vitro behaviour of plasmatocytes from host Heliothis virescens caused by Campoletis sonorensis polydnavirus. J Ins Physiol 33:143–153
Dover BA, Davies DH, Strand MR, Gray RS, Keeley LL, Vinson SB (1987) Ecdysteroid-titre reduction and developmental arrest of last-instar Heliothis virescens larvae by calyx fluid from the parasitoid Campoletis sonorensis. J Ins Physiol 33:333–338
Edson KM, Vinson SB, Stoltz DB, Summers MD (1981) Virus in a parastoid wasp: suppression of the cellular immue response in the parasitoid's host. Science 211:582–583
Essawy M, Maleville A, Brehélin M (1985) The hemocytes of Heliothis armigera: ultrastructure, functions and evolution in the course of larval development. J Morphol 186:255–264
Fleming JGW, Blissard GW, Summers MD, Vinson SB (1983) Expression of Campoletis sonorensis virus in the parasitized host, Heliothis virescens. J Virol 48:74–78
Götz P (1986) Encapsulation in arthropods. In: Brehélin M (ed) Immunity in invertebrates. Springer, Berlin Heidelberg New York, pp 153–170
Grégoire Ch, Goffinet G (1979) Controversies about the coagulocyte. In: Gupta AP (ed) Insect hemocytes. Cambridge University Press, Cambridge London New York, pp 189–229
Gupta AP (1979) Hemocyte types: their structures, synonymies, interrelationships and taxonomic significance. In: Gupta AP (ed) Insect hemocytes. Cambridge University Press, Cambridge London New York, pp 86–127
Guzo D, Stoltz DB (1985) Obligatory multiparasitism in the tussock moth, Orgyia leucostigma. Parasitology 90:1–10
Guzo D, Stoltz DB (1987) Observations on cellular immunity and parasitism in the tussock moth. J Ins Physiol 33:19–31
Kitano H (1986) The role of Apanteles glomeratus venom in the defensive response of its host, Pieris rapae crucivora. J Ins Physiol 32:369–375
Krell PJ, Summers MD, Vinson SB (1982) Virus with a multipartite superhelical genome from the ichneumonid parasitoid, Campoletis sonorensis. J Virol 43:859–870
Pathak JPN (1986) Haemogram and its endocrine control in insects. In: Brehélin M (ed) Immunity in invertebrates. Springer, Berlin Heidelberg New York, pp 49–59
Pringle JWS (1938) Proprioception in insects. J Exp Biol 15:101–103
Ratcliffe NA, Rowley AF (1979) Role of hemocytes in defense against biological agents. In: Gupta AP (ed) Insect hemocytes. Cambridge University Press, Cambridge London New York, pp 331–441
Ratner S, Schroit AJ, Vinson SB, Fidler IJ (1986) Analogous recognition of phospholoipids by insect phagocytes and mammalian macrophages (42339). Proc Soc Exp Biol Med 182:272–276
Ross DR, Dunn PE (1986) Effects of parasitization by Cotesia congregata on the antibacterial response on Manduca sexta larvae. In: Samson RA, Vlak JM, Peters D (eds) Fundamental and applied aspects of invertebrate pathology. Society of Invertebrate Pathology, pp 77–80
Stoltz DB, Guzo D (1986) Apparent haemocytic transformations associated with parasitoid-induced inhibition of immunity in Malacosoma disstria larvae. J Ins Physiol 32:377–388
Stoltz DB, Vinson SB (1979a) Viruses and parasitism in insects. Adv Virus Res 24:125–171
Stoltz DB, Vinson SB (1979b) Penetration into caterpillar cells of virus-like particles injected during oviposition by parasitoid ichneumonid wasps. Can J Microbiol 25:207–216
Stoltz DB, Krell P, Summers MD, Vinson SB (1984) Polydnaviridae — a proposed family of insect viruses with segmented, double-stranded, circular DNA genomes. Intervirology 21:1–4
Tanaka T (1987) Effect of the venom of the endoparasitoid, Apanteles kariyai Watanabe, on the cellular defence reaction of the host, Pseudaletia separata Walker. J Ins Physiol 33:413–420
Vinson SB (1972) Effect of the parasitoid, Campoletis sonorensis on the growth of its host Heliothis virescens. J Ins Physiol 18:1509–1514
Webb BA, Dahlman DL (1985) Developmental pathology of Heliothis virescens larvae parasitized by Microplitis croceipes: parasite-mediated host developmental arrest. Arch Ins Biochem Physiol 2:131–143
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davies, D.H., Vinson, S.B. Interference with function of plasmatocytes of Heliothis virescens in vivo by calyx fluid of the parasitoid Campoletis sonorensis . Cell Tissue Res. 251, 467–475 (1988). https://doi.org/10.1007/BF00215856
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00215856