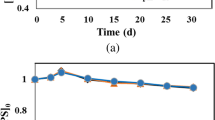
Abstract
In situ chemical oxidation (ISCO) is an effective technology for the cleanup of contaminated sites by organic compounds, such as hydrocarbons. Such remediation involves the introduction of a chemical oxidant into the subsurface in order to transform organic pollutants, present in groundwater or soil, into less harmful chemical species. Commonly applied oxidants are permanganate, hydrogen peroxide, ozone, and persulphate.
To implement the ISCO effectively, an enhancement of the contact oxidant-contaminant is required. This can be difficult in many soils and aquifers where natural features can result in significant heterogeneities in the flow of water, or in which the presence of natural reducing substances can generate non-productive reactions that consume oxidants, thus compromising their adequate distribution and efficiency. In this regard, a detailed previous knowledge of some morphological and physicochemical properties of soils and aquifers to be treated will contribute to optimize the ISCO treatments.
A present case study is currently being developed on a site with particular features that significantly influence the applicability of ISCO in any reagents. This features include: the irregular distribution of pollutants, high spatial and temporal variability of water table, generally low permeability as well as a remarkable heterogeneity of the main physicochemical soil properties (salinity, aeration, grain size or organic matter), both in the vertical and the horizontal distribution.
This chapter provides a short review of the main challenges associated to an in situ chemical oxidation treatment, specifically referred to as a site that represents a significant source of inefficiency in its application, focusing on the main issues that need to be accounted for the design of such treatment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Watts RJ, Stanton PC (1999) Mineralization of sorbed and napl-phase hexadecane by catalyzed hydrogen peroxide. Water Res 33:1405–1414
Watts RJ, Stanton PC, Howsawkeng J, Teel AL (2002) Mineralization of a sorbed polycyclic aromatic hydrocarbon in two soils using catalyzed hydrogen peroxide. Water Res 36(17):4283–4292
Baciocchi R, Boni MR, D’Aprile L (2003) Hydrogen peroxide lifetime as an indicator of the efficiency of 3-chlorophenol Fenton’s and Fenton-like oxidation in soils. J Hazard Mater B96:305–329
Watts RJ, Teel AL (2005) Chemistry of modified Fenton’s reagent (catalyzed H2O2 propagations—CHP) for in situ soil and groundwater remediation. J Environ Eng 131(4):612–622
Kong SH, Watts RJ, Choi JH (1998) Treatment of petroleum-contaminated soils using iron mineral catalyzed hydrogen peroxide. Chemosphere 37(8):1473–1482
De Souza e Silva PT, da Silva V, de Barros-Neto B, Simonnot M-O (2009) Potassium permanganate oxidation of phenanthrene and pyrene in contaminated soils. J Hazard Mater 168(2–3):1269–1273
Kislenko VN, Berlin AA, Litovchenko NV (1996) Kinetics of the oxidation of organic substances by persulfate in the presence of variable-valence metal ions. Kinet Catal 37:767–774
Block PA, Brown RA, Robinson D (2004) Novel activation technologies for sodium persulfate in situ chemical oxidation. In: Proceedings of the 4th international conference on the remediation of chlorinated and recalcitrant compounds
Boulos N, Carvel D, Mucssig J (2008) Ex-situ and in-situ remediation with activated persulfate. US Patent application 2008/0272063
Liang C, Huang CF, Chen YJ (2008) Potential for activated persulfate degradation of BTEX contamination. Water Res 42(15):4091–4100
Liang C, Lee I (2008) In situ iron activated persulfate oxidative fluid sparging treatment of TCE contamination. A proof of concept study. J Contam Hydrol 100(3–4):91–100
Romero A, Santos A, Vicente F, González C (2010) Diuron abatement using activated persulphate: effect of pH, Fe(II) and oxidant dosage. Chem Eng J 162(1):257–265
Nam K, Kukor JJ (2000) Combined ozonation and biodegradation for remediation of mixtures of polycyclic aromatic hydrocarbons in soil. Biodegradation 11:1–9
O’Mahony MM, Dobson ADW, Barnes JD, Singleton I (2006) The use of ozone in the remediation of polycyclic aromatic hydrocarbon contaminated soil. Chemosphere 63(2):307–314
Yu DY, Kang N, Bae W, Banks MK (2007) Characteristics in oxidative degradation by ozone for saturated hydrocarbons in soil contaminated with diesel fuel. Chemosphere 66(5):799–807
Rivas J, Gimeno O, de la Calle RG, Beltrán FJ (2009) Ozone treatment of PAH contaminated soils: operating variables effect. J Hazard Mater 169(1–3):509–515
Pierpoint AC, Hapeman CJ, Torrents A (2003) Ozone treatment of soil contaminated with aniline and trifluralin. Chemosphere 50:1025–1034
Bennedsen LR (2011) Activated peroxygens for remediation of contaminated soil and groundwater. Ph.D. Thesis
CityChlor (2013) Code of good practice: in situ chemical oxidation. http://www.citychlor.eu/sites/default/files/code_of_good_practice_isco.pdf. Accessed 04 Dec 2013
Huling SG, Pivetz BE (2006) In situ chemical oxidation. Engineering Issue. Prepared for the U.S. EPA. EPA/600/R-06/072. August
EPA (1998) In situ remediation technology: in situ chemical oxidation. Office of Solid Waste and Emergency Response, 542-R-98-008, September
ESTCP (1999) Technology status review: in situ oxidation. November
ITRC (The Interstate Technology & Regulatory Council) (2001) Technical and regulatory guidance for in situ chemical oxidation of contaminated soil and groundwater
GeoSyntec Consultants Inc (2004) Assessing the feasibility of DNAPL source zone remediation: review of case studies. Contract Report CR-04-002-ENV for NAVFAC, May
EPA (2004) DNAPL remediation: selected projects approaching regulatory closure. Office of Solid Waste and Emergency Response, 542-R-04-016, December
ITRC (The Interstate Technology & Regulatory Council) (2005) Technical and regulatory guidance for in situ chemical oxidation of contaminated soil and groundwater, 2nd. ed. Washington DC
McGuire TM, McDade JM, Newell CJ (2006) Performance of DNAPL source depletion technologies at 59 chlorinated solvent-impacted sites. Ground Water Monit R 26(1):73–84
Johnson et al for ESTCP (2007) Critical evaluation of state-of-the-art in situ thermal treatment technologies for DNAPL source zone treatment (ER-03 14) project fact sheet. http://www.estcp.org/Technology/ER-03 1 4-FS.cfm. Accessed 29 Aug
Siegrist RL, Crimi M, Simpkin TJ (2011) In situ chemical oxidation for groundwater remediation series: SERDP ESTCP environmental remediation technology, vol 3, first edition, p 678
Baciocchi R (2013) Principles, developments, and design criteria of in situ chemical oxidation. Water Air Soil Pollut 224(12):1–11
Krembs FJ, Siegrist RL, Crimi ML, Furrer RF, Petri BG (2010) ISCO for groundwater remediation: analysis of field applications and performance. Ground Water Monit R 30(4):42–53
APAT (2005) Protocol for ISCO application (In Italian). http://www.isprambiente.gov.it/files/temi/tec-protocolli-luglio-2005-protocollo-isco.pdf. Accessed 04 Dec 2013
Tyre BW, Watts RJ, Miller GC (1991) Treatment of four biorefractory contaminants in soils using catalyzed hydrogen peroxide. J Environ Qual 20:832–838
Watts RJ, Dilly SE (1996) Evaluation of iron catalysts for the Fenton-like remediation of diesel-contaminated soils. J Hazard Mater 51:209–224
Kakarla PKC, Watts RJ (1997) Depth of Fenton-like oxidation in remediation of surface soil. J Environ Eng 123:11–17
Watts RJ, Haller DR, Jones AP, Teel AL (2000) A foundation for the risk-based treatment of gasoline-contaminated soils using modified Fenton’s reactions. J Hazard Mater 76:73–89
Xu P, Achari G, Mahmoud M, Joshi RC (2006) Application of Fenton’s reagent to remediate diesel contaminated soils. Pract Period Hazard Toxic Radioact Waste Manage 10:19–27
Ndjou’ou AC, Cassidy D (2006) Surfactant production accompanying the modified Fenton oxidation of hydrocarbons in soil. Chemosphere 65:1610–1615
Afanas’ev IB (1989) Superoxide ion: chemistry and biological implications. CRC Press, Boca Raton, FL, p 296
De Laat J, Gallard H (1999) Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: mechanism and kinetic modeling. Environ Sci Technol 33:2726–2732
Lou JC, Lee SS (1995) Chemical oxidation of BTX using Fenton’s reagent. Hazard Mater 12(2):185–193
Lin SH, Lo CC (1997) Fenton process for treatment of desizing wastewater. Water Res 31:2050–2056
Tsitonaki A (2008) Treatment trains for the remediation of aquifers polluted with MTBE and other xenobiotic compounds. PhD Thesis
Watts RJ, SERDP (2006) Improved understanding of fenton-like reactions for the in situ remediation of contaminated groundwater including treatment of sorbed contaminants and destruction of DNAPLs
Bossmann SH, Oliveros E, Göb S, Siegwart S, Dahlen EP, Payawan LM, Matthias S, Wörner M, Braun AM (1998) New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J Phys Chem A 102:5542–5550
Smith BA, Teel AL, Watts RJ (2004) Identification of the reactive oxygen species responsible for carbon tetrachloride degradation in modified Fenton’s systems. Environ Sci Technol 38(20):5465–5469
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminated destruction based on the Fenton reaction and related chemistry. Criti Rev Environ Sci Technol 36:1–84
Baciocchi R, Ciotti C, Cleriti G, Innocenti I, Nardella A (2010) Design of in situ Fenton oxidation based on the integration of experimental and numerical modeling. J Adv Oxid Technol 13(2):153–161
Tarr M, Lindsay ME (1998) Role of dissolved organic matter in Fenton degradation of hydrophobic pollutant. Abstract presented at the 19th annual meeting of SETAC, Charlotte, NC, USA
Gates DD, Siegrist RL (1995) In situ chemical oxidation of trichloroethylene using hydrogen peroxide. J Environ Eng 121:39–644
Bissey LL, Smith JL, Watts RJ (2006) Soil organic matter–hydrogen peroxide dynamics in the treatment of contaminated soils and groundwater using catalyzed H2O2 propagations (modified Fenton’s reagent). Water Res 40:2477–2484
Bogan BW, Trbovic V (2003) Effect of sequestration on PAH degradability with Fenton’s reagent: roles of total organic carbon, humin, and soil porosity. J Hazard Mater 100:285–300
Vicente F, Rosas JM, Santos A, Romero A (2011) Improvement soil remediation by using stabilizers and chelating agents in a Fenton-like process. Chem Eng J 172:689–697
Huling SG, Arnold RG, Sierka RA, Miller MR (2001) Influence of peat on Fenton oxidation. Water Res 35(7):1687–1694
Kiwi J, Lopez A, Nadtochenko V (2000) Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl-). Environ Sci Technol 34:2162–2168
Siedlecka EM, Wieckowska A, Stepnowski P (2007) Influence of inorganic ions on MTBE degradation by Fenton’s reagent. J Hazard Mater 147:497–502
De Laat J, Le GT, Legube B (2004) A comparative study of the effects of chloride, sulfate, and nitrate ions on the rates of decomposition of H2O2 and organic compounds by Fe(II)/H2O2 and Fe(III)/H2O2. Chemosphere 55:715–723
Lipczynska-Kochany E, Sprah G, Harms S (1995) Influence of some groundwater and surface waters on the degradation of 4-chlorophenol by the Fenton reaction. Chemosphere 30:9–20
Tang WZ, Huang CP (1996) 2,4-dichlorophenol oxidation kinetics by Fenton’s reagent. Environ Technol 17:1371–1378
Watts RJ, Finn DD, Cutler LM, Schmidt JT, Teel AL (2007) Enhanced stability of hydrogen peroxide in the presence of subsurface solids. J Contam Hydrol 91:312–326
Sun Y, Pignatello JJ (1992) Chemical treatment of pesticide wastes: evaluation of Fe(III) chelates for catalyzed hydrogen peroxide oxidation of 2,4-D at circumneutral pH. J Agric Food Chem 40:322–327
Tandy S, Ammann A, Schulin R, Nowack B (2006) Biodegradation and speciation of residual SS-ethylenediaminedisuccinic acid (EDDS) in soil solution after soil washing. Environ Pollut 142:191–199
Ramo J (2003) Hydrogen peroxide-metals-chelating agents: interactions and analytical techniques. Oulu University Press, Oulu
Jones PW, Williams DR (2002) Chemical speciation simulation used to assess the efficiency of environment-friendly EDTA alternative for use in pulp and paper industry. Inorg Chim Acta 339:41–50
Tarr MA, Wei B, Zheng W, Xu G (2002) Cyclodextrin-modified fenton oxidation for in situ remediation. In: Proceedings, third international conference on remediation of chlorinated and recalcitrant compounds, Monterey, CA, USA, May 20–23, Paper 2C-17
Lindsey ME, Xu G, Lu J, Tarr MA (2003) Enhanced Fenton degradation of hydrophobic organics by simultaneous iron and pollutant complexation with cyclodextrins. Sci Total Environ 307:215–229
Jazdanian AD, Fieber LL, Tisoncik D, Huang KC, Mao F, Dahmani A, (2004) Chemical oxidation of chloroethanes and chloroethenes in a rock/groundwater system proceedings. In: Fourth international conference on remediation of chlorinated and recalcitrant compounds, Monterey, CA, USA, May 24–27, Paper 2F-01
Kang N, Hua I (2005) Enhanced chemical oxidation of aromatic hydrocarbons in soil systems. Chemosphere 61:909–922
The Colorado Department of Labor and Employment Division of Oil and Public Safety (2007) Petroleum hydrocarbon remediation by in situ chemical oxidation at Colorado Sites
In Situ Remediation Reagents Injection Working Group (2009) Subsurface injection of in situ remedial reagents (ISRRs) within the Los Angeles Regional Water Quality Control Board Jurisdiction
Rosansky S et al (2013a) Best practices for injection and distribution of amendments. Technical report TR-NAVFAC-EXWC-EV-1303
Ciotti C (2008) Advanced oxidation processes as innovative technologies for the remediation of contaminated sites. Ph.D. Thesis. University of Rome Tor Vergata
Regenesis (2007) Principles of chemical oxidation technology for the remediation of groundwater and soil, RegenOx™ design and application manual – Version 2.0. San Clemente, CA, USA
New Jersey Department of Environmental Protection (2011) Technical guidance for preparation and submission of a conceptual site model
British Columbia (2005) Technical guidance on contaminated sites. Checklist for reviewing a detailed site investigation
Rosansky S (2013) Design and quality assurance/quality control considerations for in situ chemical oxidation. Technical Memorandum TM-NAVFAC EXWC-EV-1302
Barcelona MJ, Holm T (1991) Oxidation–reduction capacities of aquifer solids. Environ Sci Technol 25(9):1565–1572
Powell RM, Callaway RW, Michalowski JT, Vandegrift SA, White MV (1988) Comparison of methods to determine oxygen demand for bioremediation of a fuel contamination of a fuel contaminated aquifer. Int J Environ Anal Chem 34:253–263
Lee W, Batchelor B (2003) Reductive capacity of natural reductants. Environ Sci Tech 37:535–541
Korom SF, McFarland MJ, Sims RC (1996) Reduced sediments: a factor in the design of subsurface oxidant delivery systems. GWMR, Winter 1996, pp 100–105
Pedersen JK, Bjerg PL, Cheristensen TH (1991) Correlation of nitrate profiles with groundwater and sediment characteristics in a shallow sandy aquifer. J Hydrol 124:263–277
IGME (1987) Mapa Geológico de España. Escala 1:50.000. San Fernando. Segunda Serie. Primera Edición. Madrid.
IGME (1990) Mapa Geológico de España. Escala 1:50.000. Chiclana de la Frontera. Segunda Serie. Primera Edición. Madrid
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
del Reino, S. et al. (2014). In Situ Chemical Oxidation Based on Hydrogen Peroxide: Optimization of Its Application to an Hydrocarbon Polluted Site. In: Jiménez, E., Cabañas, B., Lefebvre, G. (eds) Environment, Energy and Climate Change I. The Handbook of Environmental Chemistry, vol 32. Springer, Cham. https://doi.org/10.1007/698_2014_272
Download citation
DOI: https://doi.org/10.1007/698_2014_272
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12906-8
Online ISBN: 978-3-319-12907-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)