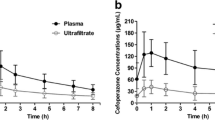
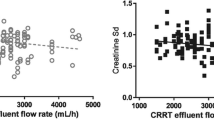
Abstract
Continuous renal replacement therapy (CRRT), particularly continuous venovenous haemofiltration (CVVH) and continuous venovenous haemodiafiltration (CVVHDF), are gaining increasing relevance in routine clinical management of intensive care unit patients. The application of CRRT, by leading to extracorporeal clearance (CLCRRT), may significantly alter the pharmacokinetic behaviour of some drugs. This may be of particular interest in critically ill patients presenting with life-threatening infections, since the risk of underdosing with antimicrobial agents during this procedure may lead to both therapeutic failure and the spread of breakthrough resistance. The intent of this review is to discuss the pharmacokinetic principles of CLCRRT of antimicrobial agents during the application of CVVH and CVVHDF and to summarise the most recent findings on this topic (from 1996 to December 2006) in order to understand the basis for optimal dosage adjustments of different antimicrobial agents.
Removal of solutes from the blood through semi-permeable membranes during RRT may occur by means of two different physicochemical processes, namely, diffusion or convection. Whereas intermittent haemodialysis (IHD) is essentially a diffusive technique and CVVH is a convective technique, CVVHDF is a combination of both. As a general rule, the efficiency of drug removal by the different techniques is expected to be CVVHDF > CVVH > IHD, but indeed CLCRRT may vary greatly depending mainly on the peculiar physicochemical properties of each single compound and the CRRT device’s characteristics and operating conditions. Considering that RRT substitutes for renal function in clearing plasma, CLCRRT is expected to be clinically relevant for drugs with dominant renal clearance, especially when presenting a limited volume of distribution and poor plasma protein binding. Consistently, CLCRRT should be clinically relevant particularly for most hydrophilic antimicrobial agents (e.g. β-lactams, aminoglycosides, glycopeptides), whereas it should assume much lower relevance for lipophilic compounds (e.g. fluoroquinolones, oxazolidinones), which generally are nonrenally cleared. However, there are some notable exceptions: ceftriax-one and oxacillin, although hydrophilics, are characterised by primary biliary elimination; levofloxacin and ciprofloxacin, although lipophilics, are renally cleared. As far as CRRT characteristics are concerned, the extent of drug removal is expected to be directly proportional to the device’s surface area and to be dependent on the mode of replacement fluid administration (predilution or postdilution) and on the ultrafiltration and/or dialysate flow rates applied.
Conversely, drug removal by means of CVVH or CVVHDF is unaffected by the drug size, considering that almost all antimicrobial agents have molecular weights significantly lower (<2000Da) than the haemofilter cut-off (30 000–50 000Da). Drugs that normally have high renal clearance and that exhibit high CLCRRT during CVVH or CVVHDF may need a significant dosage increase in comparison with renal failure or even IHD. Conversely, drugs that are normally nonrenally cleared and that exhibit very low CLCRRT during CVVH or CVVHDF may need no dosage modification in comparison with normal renal function. Bearing these principles in mind will almost certainly aid the management of antimicrobial therapy in critically ill patients undergoing CRRT, thus containing the risk of inappropriate exposure. However, some peculiar pathophys-iological conditions occurring in critical illness may significantly contribute to further alteration of the pharmacokinetics of antimicrobial agents during CRRT (i.e. hypoalbuminaemia, expansion of extracellular fluids or presence of residual renal function). Accordingly, therapeutic drug monitoring should be considered a very helpful tool for optimising drug exposure during CRRT.




















Similar content being viewed by others
References
Ronco C, Bellomo R, Ricci Z. Continuous renal replacement therapy in critically ill patients. Nephrol Dial Transplant 2001; 16 Suppl. 5: 67–72
Oda S, Hirasawa H, Shiga H, et al. Continuous hemofiltration/hemodiafiltration in critical care. Ther Apher 2002; 6: 193–8
Russell JA. Management of sepsis. N Engl J Med 2006; 355: 1699–713
Graziani G, Bordone G, Bellato V, et al. Role of the kidney in plasma cytokine removal in sepsis syndrome: a pilot study. J Nephrol 2006; 19: 176–82
Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000; 356: 26–30
Pea F, Viale P, Furlanut M. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet 2005; 44: 1009–34
Pea F, Viale P. The antimicrobial therapy puzzle: could pharma-cokinetic-pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients? Clin Infect Dis 2006; 42: 1764–71
Joy MS, Matzke GR, Armstrong DK, et al. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother 1998; 32: 362–75
Mouton JW, Touzw DJ, Horrevorts AM, et al. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin Pharmacokinet 2000; 39: 185–201
Bergan T, Engeset A, Olszewski W, et al. Extravascular penetration of highly protein-bound flucloxacillin. Antimicrob Agents Chemother 1986; 30: 729–32
Occhipinti DJ, Pendland SL, Schoonover LL, et al. Pharmacokinetics and pharmacodynamics of two multiple-dose piperacillin-tazobactam regimens. Antimicrob Agents Chemother 1997; 41: 2511–7
Barbhaiya RH, Forgue ST, Gleason CR, et al. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother 1992; 36: 552–7
Nakayama I, Akieda Y, Yamaji E, et al. Single- and multiple-dose pharmacokinetics of intravenous cefpirome (HR810) to healthy volunteers. J Clin Pharmacol 1992; 32: 256–66
Mouton JW, Horrevorts AM, Mulder PG, et al. Pharmaco-kinetics of ceftazidime in serum and suction blister fluid during continuous and intermittent infusions in healthy volunteers. Antimicrob Agents Chemother 1990; 34: 2307–11
Meyers BR, Srulevitch ES, Jacobson J, et al. Crossover study of the pharmacokinetics of ceftriaxone administered intravenously or intramuscularly to healthy volunteers. Antimicrob Agents Chemother 1983; 24: 812–4
Seddon M, Wise R, Gillett AP, et al. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother 1980; 18: 240–2
Winslade NE, Adelman MH, Evans EJ, et al. Single-dose accumulation pharmacokinetics of tobramycin and netilmicin in normal volunteers. Antimicrob Agents Chemother 1987; 31: 605–9
Wenk M, Spring P, Vozeh S, et al. Multicompartment pharma-cokinetics of netilmicin. Eur J Clin Pharmacol 1979; 16: 331–4
Golper TA, Noonan HM, Elzinga L, et al. Vancomycin pharma-cokinetics, renal handling, and nonrenal clearances in normal human subjects. Clin Pharmacol Ther 1988; 43: 565–70
Healy DP, Polk RE, Garson ML, et al. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob Agents Chemother 1987; 31: 393–7
Wilson AP. Clinical pharmacokinetics of teicoplanin. Clin Phar-macokinet 2000; 39: 167–83
Carver PL, Nightingale CH, Quintiliani R, et al. Pharmaco-kinetics of single- and multiple-dose teicoplanin in healthy volunteers. Antimicrob Agents Chemother 1989; 33: 82–6
Gonzalez MA, Moranchel AH, Duran S, et al. Multiple-dose ciprofloxacin dose ranging and kinetics. Clin Pharmacol Ther 1985; 37: 633–7
Chow AT, Fowler C, Williams RR, et al. Safety and pharma-cokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob Agents Che-mother 2001; 45: 2122–5
Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet 1997; 32: 101–19
Stass H, Dalhoff A, Kubitza D, et al. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother 1998; 42: 2060–5
Lubasch A, Keller I, Borner K, et al. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother 2000; 44: 2600–3
Guay DR, Opsahl JA, McMahon FG, et al. Safety and pharma-cokinetics of multiple doses of intravenous ofloxacin in healthy volunteers. Antimicrob Agents Chemother 1992; 36: 308–12
Lode H, Hoffken G, Olschewski P, et al. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob Agents Chemother 1987; 31: 1338–42
MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Grampositive infections. J Antimicrob Chemother 2003; 51 Suppl. 2: ii17–25
Slatter JG, Stalker DJ, Feenstra KL, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab Dispos 2001; 29: 1136–45
Bekersky I, Fielding RM, Dressler DE, et al. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother 2002; 46: 828–33
Dupont B. Overview of the lipid formulations of amphotericin B. J Antimicrob Chemother 2002; 49 Suppl. 1: 31–6
Ripa S, Ferrante L, Prenna M. Pharmacokinetics of fluconazole in normal volunteers. Chemotherapy 1993; 39: 6–12
Bouman CS, van Kan HJ, Koopmans RP, et al. Discrepancies between observed and predicted continuous venovenous hemofiltration removal of antimicrobial agents in critically ill patients and the effects on dosing. Intensive Care Med 2006; 32: 2013–9
Golper TA. Update on drug sieving coefficients and dosing adjustments during continuous renal replacement therapies. Contrib Nephrol 2001: 349–53
Golper TA, Marx MA. Drug dosing adjustments during continuous renal replacement therapies. Kidney Int Suppl 1998; 66: S165–8
Pea F, Brollo L, Lugano M, et al. Therapeutic drug monitoring-guided high teicoplanin dosage regimen required to treat a hypoalbuminaemic renal transplant patient undergoing continuous venovenous hemofiltration. Ther Drug Monit 2001; 23: 587–8
Arzuaga A, Maynar J, Gascon AR, et al. Influence of renal function on the pharmacokinetics of piperacillin/tazobactam in intensive care unit patients during continuous venovenous hemofiltration. J Clin Pharmacol 2005; 45: 168–76
Thalhammer F, Schenk P, Burgmann H, et al. Single-dose pharmacokinetics of meropenem during continuous venovenous hemofiltration. Antimicrob Agents Chemother 1998; 42: 2417–20
Tegeder I, Neumann F, Bremer F, et al. Pharmacokinetics of meropenem in critically ill patients with acute renal failure undergoing continuous venovenous hemofiltration. Clin Pharmacol Ther 1999; 65: 50–7
Ververs TF, van Dijk A, Vinks SA, et al. Pharmacokinetics and dosing regimen of meropenem in critically ill patients receiving continuous venovenous hemofiltration. Crit Care Med 2000; 28: 3412–6
Krueger WA, Neeser G, Schuster H, et al. Correlation of mer-openem plasma levels with pharmacodynamic requirements in critically ill patients receiving continuous veno-venous hemofiltration. Chemotherapy 2003; 49: 280–6
Giles LJ, Jennings AC, Thomson AH, et al. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit Care Med 2000; 28: 632–7
Robatel C, Decosterd LA, Biollaz J, et al. Pharmacokinetics and dosage adaptation of meropenem during continuous venovenous hemodiafiltration in critically ill patients. J Clin Pharmacol 2003; 43: 1329–40
Valtonen M, Tiula E, Backman JT, et al. Elimination of meropenem during continuous veno-venous haemofiltration and haemodiafiltration in patients with acute renal failure. J Antimicrob Chemother 2000; 45: 701–4
Isla A, Maynar J, Sanchez-Izquierdo JA, et al. Meropenem and continuous renal replacement therapy: in vitro permeability of 2 continuous renal replacement therapy membranes and influence of patient renal function on the pharmacokinetics in critically ill patients. J Clin Pharmacol 2005; 45: 1294–304
Tegeder I, Bremer F, Oelkers R, et al. Pharmacokinetics of imipenem-cilastatin in critically ill patients undergoing continuous venovenous hemofiltration. Antimicrob Agents Che-mother 1997; 41: 2640–5
Fish DN, Teitelbaum I, Abraham E. Pharmacokinetics and pharmacodynamics of imipenem during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 2005; 49: 2421–8
Meyer B, Ahmed el Gendy S, Delle Karth G, et al. How to calculate clearance of highly protein-bound drugs during continuous venovenous hemofiltration demonstrated with fluclox-acillin. Kidney Blood Press Res 2003; 26: 135–40
Capellier G, Cornette C, Boillot A, et al. Removal of piperacillin in critically ill patients undergoing continuous venovenous hemofiltration. Crit Care Med 1998; 26: 88–91
van der Werf TS, Mulder PO, Zijlstra JG, et al. Pharmaco-kinetics of piperacillin and tazobactam in critically ill patients with renal failure, treated with continuous veno-venous hemofiltration (CVVH). Intensive Care Med 1997; 23: 873–7
Valtonen M, Tiula E, Takkunen O, et al. Elimination of the piperacillin/tazobactam combination during continuous ve-novenous haemofiltration and haemodiafiltration in patients with acute renal failure. J Antimicrob Chemother 2001; 48: 881–5
Mueller SC, Majcher-Peszynska J, Hickstein H, et al. Pharma-cokinetics of piperacillin-tazobactam in anuric intensive care patients during continuous venovenous hemodialysis. Antimicrob Agents Chemother 2002; 46: 1557–60
Allaouchiche B, Breilh D, Jaumain H, et al. Pharmacokinetics of cefepime during continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 1997; 41: 2424–7
Malone RS, Fish DN, Abraham E, et al. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 2001; 45: 3148–55
Isla A, Gascon AR, Maynar J, et al. Cefepime and continuous renal replacement therapy (CRRT): in vitro permeability of two CRRT membranes and pharmacokinetics in four critically ill patients. Clin Ther 2005; 27: 599–608
Van der Werf TS, Fijen JW, Van de Merbel NC, et al. Pharma-cokinetics of cefpirome in critically ill patients with renal failure treated by continuous veno-venous hemofiltration. Intensive Care Med 1999; 25: 1427–31
Banyai M, Thalhammer F, El-Menyawi I, et al. Pharmaco-kinetics of cefpirome during continuous venovenous hemofiltration: rationale for an 8-hour dosing interval. Clin Pharmacol Ther 2000; 67: 368–72
Sato T, Okamoto K, Kitaura M, et al. The pharmacokinetics of ceftazidime during hemodiafiltration in critically ill patients. Artif Organs 1999; 23: 143–5
Matzke GR, Frye RF, Joy MS, et al. Determinants of ceftazidime clearance by continuous venovenous hemofiltration and continuous venovenous hemodialysis. Antimicrob Agents Chemother 2000; 44: 1639–44
Traunmuller F, Schenk P, Mittermeyer C, et al. Clearance of ceftazidime during continuous venovenous haemofiltration in critically ill patients. J Antimicrob Chemother 2002; 49: 129–34
Mariat C, Venet C, Jehl F, et al. Continuous infusion of ceftazidime in critically ill patients undergoing continuous venovenous haemodiafiltration: pharmacokinetic evaluation and dose recommendation. Crit Care 2006; 10: R26
Kroh UF, Lennartz H, Edwards DJ, et al. Pharmacokinetics of ceftriaxone in patients undergoing continuous veno-venous hemofiltration. J Clin Pharmacol 1996; 36: 1114–9
Matzke GR, Frye RF, Joy MS, et al. Determinants of ceftriaxone clearance by continuous venovenous hemofiltration and hemodialysis. Pharmacotherapy 2000; 20: 635–43
Syka M, Markantonis SL, Mathas C, et al. A pilot study of netilmicin pharmacokinetics during continuous venovenous hemodiafiltration. J Clin Pharmacol 2005; 45: 477–81
Meyer B, Traunmuller F, Hamwi A, et al. Pharmacokinetics of teicoplanin during continuous hemofiltration with a new and a 24-h used highly permeable membrane: rationale for therapeutic drug monitoring-guided dosage. Int J Clin Pharmacol Ther 2004; 42: 556–60
Yagasaki K, Gando S, Matsuda N, et al. Pharmacokinetics of teicoplanin in critically ill patients undergoing continuous hemodiafiltration. Intensive Care Med 2003; 29: 2094–5
Joy MS, Matzke GR, Frye RF, et al. Determinants of vancomycin clearance by continuous venovenous hemofiltration and continuous venovenous hemodialysis. Am J Kidney Dis 1998; 31: 1019–27
Boereboom FT, Ververs FF, Blankestijn PJ, et al. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Intensive Care Med 1999; 25: 1100–4
Uchino S, Cole L, Morimatsu H, et al. Clearance of vancomycin during high-volume haemofiltration: impact of pre-dilution. Intensive Care Med 2002; 28: 1664–7
DelDot ME, Lipman J, Tett SE. Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous haemodiafiltration. Br J Clin Pharmacol 2004; 58: 259–68
Malone RS, Fish DN, Abraham E, et al. Pharmacokinetics of levofloxacin and ciprofloxacin during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 2001; 45: 2949–54
Guenter SG, Iven H, Boos C, et al. Pharmacokinetics of levofloxacin during continuous venovenous hemodiafiltration and continuous venovenous hemofiltration in critically ill patients. Pharmacotherapy 2002; 22: 175–83
Hansen E, Bucher M, Jakob W, et al. Pharmacokinetics of levofloxacin during continuous veno-venous hemofiltration. Intensive Care Med 2001; 27: 371–5
Traunmuller F, Thalhammer-Scherrer R, Locker GJ, et al. Single-dose pharmacokinetics of levofloxacin during continuous veno-venous haemofiltration in critically ill patients. J Antimicrob Chemother 2001; 47: 229–31
Bellmann R, Egger P, Gritsch W, et al. Elimination of levofloxacin in critically ill patients with renal failure: influence of continuous veno-venous hemofiltration. Int J Clin Pharmacol Ther 2002; 40: 142–9
Fuhrmann V, Schenk P, Jaeger W, et al. Pharmacokinetics of moxifloxacin in patients undergoing continuous venovenous haemodiafiltration. J Antimicrob Chemother 2004; 54: 780–4
Fuhrmann V, Schenk P, Mittermayer C, et al. Single-dose pharmacokinetics of ofloxacin during continuous venovenous hemofiltration in critical care patients. Am J Kidney Dis 2003; 42: 310–4
Pea F, Viale P, Lugano M, et al. Linezolid disposition after standard dosages in critically ill patients undergoing continuous venovenous hemofiltration: a report of 2 cases. Am J Kidney Dis 2004; 44: 1097–102
Fiaccadori E, Maggiore U, Rotelli C, et al. Removal of linezolid by conventional intermittent hemodialysis, sustained low-efficiency dialysis, or continuous venovenous hemofiltration in patients with acute renal failure. Crit Care Med 2004; 32: 2437–42
Meyer B, Kornek GV, Nikfardjam M, et al. Multiple-dose pharmacokinetics of linezolid during continuous venovenous haemofiltration. J Antimicrob Chemother 2005; 56: 172–9
Meyer B, Thalhammer F. Linezolid and continuous venovenous hemofiltration. Clin Infect Dis 2006; 42: 435–8
Mauro LS, Peloquin CA, Schmude K, et al. Clearance of line-zolid via continuous venovenous hemodiafiltration. Am J Kidney Dis 2006; 47: e83–6
Kraft MD, Pasko DA, DePestel DD, et al. Linezolid clearance during continuous venovenous hemodiafiltration: a case report. Pharmacotherapy 2003; 23: 1071–5
Li J, Rayner CR, Nation RL, et al. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 2005; 49: 4814–5
Bellmann R, Egger P, Gritsch W, et al. Amphotericin B lipid formulations in critically ill patients on continuous veno-venous haemofiltration. J Antimicrob Chemother 2003; 51: 671–81
Bellmann R, Egger P, Djanani A, et al. Pharmacokinetics of amphotericin B lipid complex in critically ill patients on continuous veno-venous haemofiltration. Int J Antimicrob Agents 2004; 23: 80–3
Bergner R, Hoffmann M, Riedel KD, et al. Fluconazole dosing in continuous veno-venous haemofiltration (CVVHF): need for a high daily dose of 800 mg. Nephrol Dial Transplant 2006; 21: 1019–23
Yagasaki K, Gando S, Matsuda N, et al. Pharmacokinetics and the most suitable dosing regimen of fluconazole in critically ill patients receiving continuous hemodiafiltration. Intensive Care Med 2003; 29: 1844–8
Vos MC, Vincent HH, Yzerman EP. Clearance of imipenem/cilastatin in acute renal failure patients treated by continuous hemodiafiltration (CAVHD). Intensive Care Med 1992; 18: 282–5
Arzuaga A, Isla A, Gascon AR, et al. Elimination of piperacillin and tazobactam by renal replacement therapies with AN69 and polysulfone hemofilters: evaluation of the sieving coefficient. Blood Purif 2006; 24: 347–54
Thalhammer F, Siostrzonek P. Cefpirome and continuous ve-novenous hemofiltration. Intensive Care Med 2000; 26: 830
Pea F, Viale P, Damiani D, et al. Ceftazidime in acute myeloid leukemia patients with febrile neutropenia: helpfulness of continuous intravenous infusion in maximizing pharmacodynamic exposure. Antimicrob Agents Chemother 2005; 49: 3550–3
Barbot A, Venisse N, Rayeh F, et al. Pharmacokinetics and pharmacodynamics of sequential intravenous and subcutaneous teicoplanin in critically ill patients without vasopressors. Intensive Care Med 2003; 29: 1528–34
Pea F, Poz D, Viale P, et al. Which reliable pharmacodynamic breakpoint should be advised for ciprofloxacin monotherapy in the hospital setting? A TDM-based retrospective perspective. J Antimicrob Chemother 2006; 58: 380–6
Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006; 6: 589–601
Trotman RL, Williamson JC, Shoemaker DM, et al. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis 2005; 41: 1159–66
Acknowledgements
No sources of funding were used to assist in the preparation of this review. Federico Pea has been a consultant to Pfizer and Sanofi-Aventis, and has been on the speakers’ bureau for Pfizer, Sanofi-Aventis, Abbott, Bayer, Gilead, GlaxoSmithKline and Merck Sharp & Dohme. Pierluigi Viale has been a consultant to Merck Sharp & Dohme, Pfizer and Sanofi-Aventis, has been on the speakers’ bureau for Merck Sharp & Dohme, Pfizer, Sanofi-Aventis, Bayer, GlaxoSmithKline, Abbott, Gilead and Wyeth, and has received grant support from Merck Sharp & Dohme, Pfizer, Sanofi-Aventis, Bayer and GlaxoSmithKline. Mario Furlanut has received grant support from GlaxoSmithKline and Sanofi-Aventis. Federica Pavan has no potential conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pea, F., Viale, P., Pavan, F. et al. Pharmacokinetic Considerations for Antimicrobial Therapy in Patients Receiving Renal Replacement Therapy. Clin Pharmacokinet 46, 997–1038 (2007). https://doi.org/10.2165/00003088-200746120-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200746120-00003