Abstract
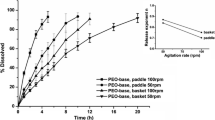
The objective of this study was to determine the influence of mechanical stresses simulating gastrointestinal contraction forces of 2.0 N (stomach) and 1.2 N (intestine) on the gel properties and drug release characteristics from sustained release swelling and eroding hydrophilic matrices during dissolution studies. Two batches of tetracycline-sustained release tablets containing hydroxypropyl methyl cellulose (HPMC) were manufactured and subjected to USP apparatus II (pH 2.2 buffer) dissolution studies. Hydrated tablets were periodically removed, placed in a petri dish, and multiple times (six cycle) compressed with a flat-ended probe (diameter 1.3 cm) on a texture analyzer at preprogrammed force of either 2.0 or 1.2 N to determine force-distance profiles and changes in drug release rate. The calculated similarity factor values showed dissimilar dissolution profiles using standard dissolution profile as a reference. The similarity factor (f 2) values were especially lower than 50 at 2.0 N and, when profiles between the two batches compressed at 1.2 and 2.0 N, were compared with each other. The changes in dissolution pattern and release rate were significantly different after 4 h of dissolution. At 8 h, tablets were fully hydrated and no force could be detected by the probe, indicating a very soft gel matrix. It appears that the contraction forces in the stomach and intestine are capable of altering drug release from modified release hydrophilic matrices during transit in the human GI tract. Accounting for these forces during dissolution can enhance predictions of in vivo drug release, achieve better in vitro and in vivo correlation, introduce improvement in dissolution methods, and better understand the critical quality attributes (CQAs) and factors in quality by design (QbD) during the product development process.






Similar content being viewed by others
REFERENCES
Larregieu CA, Benet LZ. Distinguishing between the permeability relationships with absorption and metabolism to improve BCS and BDDCS predictions in early drug discovery. Mol Pharmaceutics. 2014;11:1335–44.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20.
Yu LX, Crison JR, Amidon GL. Compartmental transit and dispersion model analysis of small intestinal transit flow in humans. Int J Pharm. 1996;140:111–8.
Sjögren E, Westergren J, Grant I, Hanisch G, Lindfors L, Lennernäs H, et al. Eur J Pharm Sci. 2013;49:679–98.
Parrott N, Lave T. Applications of physiologically based absorption models in drug discovery and development. Mol Pharm. 2008;5:760–75.
Sugano K. Fraction of a dose absorbed estimation for structurally diverse low solubility compounds. Int J Pharm. 2011;405:79–89.
Ekarat J, De Maiob V, Rondab E, Mattavelli V, Vertzonic M, Dressman JB. Eur J Pharm Sci. 2009;37:434–41.
Jantratid Ekarat and Dressman Jennifer; Biorelevant dissolution media simulating the proximal human gastrointestinal tract: an update. Dissolution Technologies, August 2009, 21–25
Vangelderen MM, Olling M, Barends DM, Meulenbelt J, Salomons P, Rauws A. The bioavailability of diclofenac from enteric coated products in healthy volunteers with normal and artificially decreased gastric acidity. Biopharm Drug Dispos. 1994;15:775–88.
Wilding IR, Coupe AJ, Davis SS. The role of gamma-scintigraphy in oral drug delivery. Adv Drug Deliv Rev. 2001;46(1–3):103–24.
Kamba M, Seta Y, Kusai A, Ikeda M, Nishimura K. A unique dosage form to evaluate the mechanical destructive force in the gastrointestinal tract. Int J Pharm. 2000;208(1–2):61–70.
Kamba M, Seta Y, Kusai A, Nishimura K. Comparison of the mechanical destructive force in the small intestine of dog and human. Int J Pharm. 2002;237(1–2):139–49.
Rubinstein A, Li VHK, Robinson JR. Gastrointestinal-physiological variables affecting performance of oral sustained release dosage forms. In Yacobi A, Halperin-Walega E (eds), Oral sustained release formulation: design and evaluation. NY, Pergamon, 1988, Chap 6.
United States Pharmacopeial (USP-NF) (2011), USP Convention Inc. 12601 Twinbrook Parkway, Rockville, MD 20852.
Durig T, Fassihi R. Evaluation of floating and sticking extended release delivery systems: an unconventional dissolution test. J Control Release. 2000;67(1):37–44.
Yang L, Johnson B, Fassihi R. Determination of continuous changes in the gel layer thickness of poly(ethylene oxide) and HPMC tablets undergoing hydration: a texture analysis study. Pharm Res. 1998;15(12):1902–6.
Jamzad S, Fassihi R. Development of a controlled release low dose class II drug—Glipizide. Int J Pharmaceutics. 2006;312:24–32.
Shah VP, Tsong Y, Sathe P, Liu JP. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15(6):889–96.
ICH guidance documents Q8 R1 2009 (pharmaceutical development revision 1), Q9 2006 (quality risk management) and Q10 2009 (pharmaceutical quality system). http://www.fda.gov/cder/guidance/.
Butler JM, Dressman J. The developability classification system: application of biopharmaceutics concepts to formulation development. J Pharm Sci. 2010;99(12):4940–54.
ACKNOWLEDGMENTS
The authors would like to acknowledge Ms. Zheng Lu for assisting in texture analysis work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to Professor M.R. Hojat, Department of Psychiatry and Human Behavior, and the Director of Jefferson Longitudinal Study, on the occasion of his 66th birthday. He is one of the pioneers in the development of the scales that measure aspects of professionalism in medicine including collaborations between physician, nurse, and pharmacist.
Rights and permissions
About this article
Cite this article
Takieddin, M., Fassihi, R. A Novel Approach in Distinguishing Between Role of Hydrodynamics and Mechanical Stresses Similar to Contraction Forces of GI Tract on Drug Release from Modified Release Dosage Forms. AAPS PharmSciTech 16, 278–283 (2015). https://doi.org/10.1208/s12249-014-0225-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0225-5