Abstract
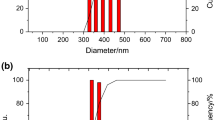
The aim of the present study was to formulate serratiopeptidase (SER)-loaded chitosan (CS) nanoparticles for oral delivery. SER is a proteolytic enzyme which is very sensitive to change in temperature and pH. SER-loaded CS nanoparticles were fabricated by ionic gelation method using tripolyphosphate (TPP). Nanoparticles were characterized for its particle size, morphology, entrapment efficiency, loading efficiency, percent recovery, and in vitro dissolution study. SER-CS nanoparticles had a particle size in the range of 400–600 nm with polydispersity index below 0.5. SER association was up to 80 ± 4.2%. SER loading and CS/TPP mass ratio were the primary parameters having direct influence on SER-CS nanoparticles. SER-CS nanoparticles were freeze dried using trehalose (20%) as a cryoprotectant. In vitro dissolution showed initial burst followed by sustained release up to 24 h. In vivo anti-inflammatory activity was carried out in rat paw edema model. In vivo anti-inflammatory activity in rat paw edema showed prolonged anti-inflammatory effect up to 32 h relative to plain SER.




Similar content being viewed by others
References
des Rieux A, Fievez V, Garinot M, Schneider Y-J, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116(1):1–27.
Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res. 2007;24(12):2198–206.
Fd C, Aj T, Dm C, Lq Z, Shi K. Preparation of insulin loaded PLGA-Hp55 nanoparticles for oral delivery. J Pharm Sci. 2007;96(2):421–7.
Tasset C, Barette N, Thysman S, Ketelslegers J-M, Lemoine D. Polyisobutylcyanoacrylate nanoparticles as sustained release system for calcitonin. J Control Release. 1995;33(1):23–30.
Santander-Ortega MJ, Csaba N, González L, Bastos-González D, Ortega-Vinuesa JL, Alonso MJ. Protein-loaded PLGA-PEO blend nanoparticles: encapsulation, release and degradation characteristics. Colloid Polym Sci. 2010;288(2):141–50.
Lin Y-H, Chang C-H, Wu Y-S, Hsu Y-M, Chiou S-F, Chen Y-J. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti- < i > Helicobacter pylori</i > therapy. Biomaterials. 2009;30(19):3332–42.
Santander-Ortega M, Bastos-Gonzalez D, Ortega-Vinuesa J, Alonso M. Insulin-loaded PLGA nanoparticles for oral administration: an in vitro physico-chemical characterization. J Biomed Nanotechnol. 2009;5(1):45–53.
Patel AR, Kulkarni S, Nandekar TD, Vavia PR. Evaluation of alkyl polyglucoside as an alternative surfactant in the preparation of peptide-loaded nanoparticles. J Microencapsul. 2008;25(8):531–40.
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Control Release. 2004;100(1):5–28.
Mazzone A, Catalani M, Costanzo M, Drusian A, Mandoli A, Russo S, et al. Evaluation of Serratia peptidase in acute or chronic inflammation of otorhinolaryngology pathology: a multicentre, double-blind, randomized trial versus placebo. J Int Med Res. 1989;18(5):379–88.
Jadav SP, Patel NH, Shah TG, Gajera MV, Trivedi HR, Shah BK. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J Pharmacol Pharmacother. 2010;1(2):116.
Fereidoni M, Ahmadiani A, Semnanian S, Javan M. An accurate and simple method for measurement of paw edema. J Pharmacol Toxicol Methods. 2000;43(1):11–4.
MAEJIMA K, MIYATA K, TOMODA K. A manganese superoxide dismutase from Serratia marcescens. Agric Biol Chem. 1983;47(7):1537–43.
Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–32.
Mao H-Q, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, et al. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70(3):399–421.
Tokumitsu H, Ichikawa H, Fukumori Y. Chitosan-gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion-droplet coalescence technique and characterization. Pharm Res. 1999;16(12):1830–5.
Mitra S, Gaur U, Ghosh P, Maitra A. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74(1):317–23.
Calvo P, Remunan-Lopez C, Vila-Jato J, Alonso M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63(1):125–32.
Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier—systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B: Biointerfaces. 2007;59(1):24–34.
Aktaş Y, Andrieux K, Alonso MJ, Calvo P, Gürsoy RN, Couvreur P, et al. Preparation and in vitro evaluation of chitosan nanoparticles containing a caspase inhibitor. Int J Pharm. 2005;298(2):378–83.
Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release. 2006;115(2):216–25.
Rudzinski WE, Aminabhavi TM. Chitosan as a carrier for targeted delivery of small interfering RNA. Int J Pharm. 2010;399(1):1–11.
Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan—TPP nanoparticles intended for gene delivery. Colloids Surf B: Biointerfaces. 2005;44(2):65–73.
Csaba N, Köping-Höggård M, Alonso MJ. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int J Pharm. 2009;382(1):205–14.
Kumbhar D, Wavikar P, Vavia P. Niosomal Gel of Lornoxicam for topical delivery: in vitro assessment and pharmacodynamic activity. AAPS PharmSciTech. 2013;14(3):1072–82.
Crystalline KM, Desoxyribonuclease I. Isolation and general properties spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950;33(4):349–62.
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688–713.
Date PV, Samad A, Devarajan PV. Freeze thaw: a simple approach for prediction of optimal cryoprotectant for freeze drying. AAPS PharmSciTech. 2010;11(1):304–13.
Acknowledgments
The authors want to acknowledge DBT and UGC for fellowship and AICTE/NAFETIC and Kusum HealthCare for proving laboratory facilities.
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mali, N., Wavikar, P. & Vavia, P. Serratiopeptidase Loaded Chitosan Nanoparticles by Polyelectrolyte Complexation: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 16, 59–66 (2015). https://doi.org/10.1208/s12249-014-0201-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0201-0