Abstract
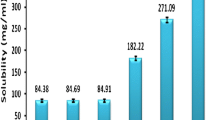
Utilization of lipid-based drug delivery systems has recently gained focus for drugs characterized by poor aqueous solubility. The improved aqueous solubility overcomes one of the main barriers that limit their bioavailability. The objective of this work was to improve the solubility and oral bioavailability of Avanafil (AVA), a recently approved second generation type 5 phospodiesterase inhibitor used for erectile dysfunction.AVA was formulated as self-nanoemulsifying drug delivery system (SNEDDS) utilizing various oils, surfactants, and cosurfactants. The solubility of AVA in various oils, surfactants, and cosurfactants was determined. Ternary phase diagram was constructed to identify stable nanoemulsion region. The prepared AVA loaded SNEDDS were assessed for optical clarity, droplet size, conductivity, and stability studies. In vitro drug release and in vivo pharmacokinetic parameters using animal model were also investigated. Results revealed that stable AVA (SNEDDS) were successfully developed with a droplet size range of 65 to 190 nm. SNEDDS composed of 25% dill oil, 55% Tween 80, and 20% propylene glycol successfully improved solubilization of AVA (over 80% within 30 min) vis-a-vis the powder AVA (35% within 30 min). In vivo pharmacokinetic showed a significant (P < 0.05) increase in Cmax, reduction in Tmax, and SNEDDS enhanced the bioavailability in the rats by 1.4-fold when compared with pure drug.




Similar content being viewed by others
References
Sanford M. Avanafil: a review of its use in patients with erectile dysfunction. Drugs Aging. 2013;30:853–62.
FDA approves Stendra for erectile dysfunction 2014.
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP), CHMP assessment report, EMA, 321885, 2013.
Ahmed OAA, Afouna MI, El-Say KM, Abdel-Naim AB, Khedr A, Banjar ZM. Optimization of self nanoemulsifying systems for the enhancement of in vivo hypoglycemic efficacy of glimepiride transdermal patches. Expert Opin Drug Deliv 2014;11:In press.
Ahmed OAA, Badr-Eldin SM, Tawfik MK, Ahmed TA, El-Say KM, Badr JM. Design and optimization of self-nanoemulsifying delivery system to enhance quercetin hepatoprotective activity in paracetamol-induced hepatotoxicity. J Pharm Sci. 2014;103:602–12.
Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine (London). 2010;5:1595–616.
Dixit RP, Nagarsenker MS. Self-nanoemulsifying granules of ezetimibe: design, optimization and evaluation. Eur J Pharm Sci. 2008;35:183–92.
Janga KY, Jukanti R, Sunkavalli S, Velpula A, Bandari S, Kandadi P, et al. In situ absorption and relative bioavailability studies of zaleplon loaded self-nanoemulsifying powders. J Microencapsul. 2013;30:161–72.
Ren F, Gao Y, Chen J, Jing Q, Yu Y. New self-nanoemulsifying drug delivery system (SNEDDS) with amphiphilic diblock copolymer methoxy poly (ethylene glycol)-block-poly (−caprolactone). Pharm Dev Technol. 2013;18:745–51.
Singh B, Bandyopadhyay S, Kapil R, Singh R, Katare O. Self-emulsifying drug delivery systems (SEDDS): formulation development, characterization, and applications. Crit Rev Ther Drug Carrier Syst. 2009;26:427–521.
Singh B, Khurana L, Bandyopadhyay S, Kapil R, Katare OO. Development of optimized self-nano-emulsifying drug delivery systems (SNEDDS) of carvedilol with enhanced bioavailability potential. Drug Deliv. 2011;18:599–612.
Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12:1561–72.
Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25:47–58.
El Maghraby GM. Self-microemulsifying and microemulsion systems for transdermal delivery of indomethacin: effect of phase transition. Colloids Surf B: Biointerfaces. 2010;75:595–600.
Hosny KM, Banjar ZM. The formulation of a nasal nanoemulsion zaleplon in situ gel for the treatment of insomnia. Expert Opin Drug Deliv. 2013;10:1033–41.
Jung J, Choi S, Cho SH, Ghim JL, Hwang A, Kim U, et al. Tolerability and pharmacokinetics of avanafil, a phosphodiesterase type 5 inhibitor: a single- and multiple-dose, double-blind, randomized, placebo-controlled, dose-escalation study in healthy Korean male volunteers. Clin Ther. 2010;32:1178–87.
Hugger ED, Novak BL, Burton PS, Audus KL, Borchardt RT. A comparison of commonly used polyethoxylated pharmaceutical excipients on their ability to inhibit P-glycoprotein activity in vitro. J Pharm Sci. 2002;91:1991–2002.
Türk M, Hils P, Helfgen B, Schaber K, Martin H-J, Wahl MA. Micronization of pharmaceutical substances by the Rapid Expansion of Supercritical Solutions (RESS): a promising method to improve bioavailability of poorly soluble pharmaceutical agents. J Supercrit Fluids. 2002;22:75–84.
Yuan JS, Ansari M, Samaan M, Acosta EJ. Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int J Pharm. 2008;349:130–43.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121.
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int J Pharm. 2010;391:203–11.
Djekic L, Primorac M. The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides. Int J Pharm. 2008;352:231–9.
Conflict of Interest
The authors state no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fahmy, U.A., Ahmed, O.A.A. & Hosny, K.M. Development and Evaluation of Avanafil Self-nanoemulsifying Drug Delivery System with Rapid Onset of Action and Enhanced Bioavailability. AAPS PharmSciTech 16, 53–58 (2015). https://doi.org/10.1208/s12249-014-0199-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0199-3