Abstract
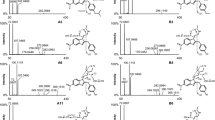
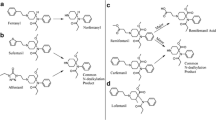
New fentanyl analogs have recently emerged as new psychoactive substances and have caused numerous fatalities worldwide. To determine if the new analogs follow the same metabolic pathways elucidated for fentanyl and known fentanyl analogs, we performed in vitro and in vivo metabolite identification studies for acetylfentanyl, acrylfentanyl, 4-fluoro-isobutyrylfentanyl, and furanylfentanyl. All compounds were incubated at 10 μM with pooled human hepatocytes for up to 5 h. For each compound, four or five authentic human urine samples from autopsy cases with and without enzymatic hydrolysis were analyzed. Data acquisition was performed in data-dependent acquisition mode during liquid chromatography high-resolution mass spectrometry analyses. Data was analyzed (1) manually based on predicted biotransformations and (2) with MetaSense software using data-driven search algorithms. Acetylfentanyl, acrylfentanyl, and 4-fluoro-isobutyrylfentanyl were predominantly metabolized by N-dealkylation, cleaving off the phenethyl moiety, monohydroxylation at the ethyl linker and piperidine ring, as well as hydroxylation/methoxylation at the phenyl ring. In contrast, furanylfentanyl’s major metabolites were generated by amide hydrolysis and dihydrodiol formation, while the nor-metabolite was minor or not detected in case samples at all. In general, in vitro results matched the in vivo findings well, showing identical biotransformations in each system. Phase II conjugation was observed, particularly for acetylfentanyl. Based on our results, we suggest the following specific and abundant metabolites as analytical targets in urine: a hydroxymethoxy and monohydroxylated metabolite for acetylfentanyl, a monohydroxy and dihydroxy metabolite for acrylfentanyl, two monohydroxy metabolites and a hydroxymethoxy metabolite for 4-fluoro-isobutyrylfentanyl, and a dihydrodiol metabolite and the amide hydrolysis metabolite for furanylfentanyl.





Similar content being viewed by others
References
European Monitoring Centre for Drugs and Drug Addiction. EMCDDA–Europol 2015 Annual Report on the implementation of Council Decision 2005/387/JHA2016 26 September 2016. Available from: http://www.emcdda.europa.eu/publications/implementation-reports/2015.
Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 1993;52(4):389–96. doi:10.1016/0024-3205(93)90152-S.
Higashikawa Y, Suzuki S. Studies on 1-(2-phenethyl)-4-(N-propionylanilino) piperidine (fentanyl) and its related compounds. VI. Structure-analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues. Forensic Toxicol. 2008;26(1):1–5. doi:10.1007/s11419-007-0039-1.
Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5(4):230–41. doi:10.1007/bf03178274.
Kronstrand R, Druid H, Holmgren P, Rajs J. A cluster of fentanyl-related deaths among drug addicts in Sweden. Forensic Sci Int. 1997;88(3):185–95. doi:10.1016/S0379-0738(97)00068-6.
Vardanyan RS, Hruby VJ. Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications. Future Med Chem. 2014;6(4):385–412. doi:10.4155/fmc.13.215.
Henderson G. Designer drugs: past history and future prospects. J Forensic Sci. 1988;33(2):569–75. doi:10.1520/JFS11976J.
Helander A, Backberg M, Beck O. Intoxications involving the fentanyl analogs acetylfentanyl, 4-methoxybutyrfentanyl and furanylfentanyl: results from the Swedish STRIDA project. Clin Toxicol (Phila). 2016;54(4):324–32. doi:10.3109/15563650.2016.1139715.
Backberg M, Beck O, Jonsson KH, Helander A. Opioid intoxications involving butyrfentanyl, 4-fluorobutyrfentanyl, and fentanyl from the Swedish STRIDA project. Clin Toxicol (Phila). 2015;53(7):609–17. doi:10.3109/15563650.2015.1054505.
McClain DA, Hug CC. Intravenous fentanyl kinetics. Clinical Pharmacology & Therapeutics. 1980;28(1):106–14. doi:10.1038/clpt.1980.138.
Goromaru T, Matsuura H, Yoshimura N, Miyawaki T, Sameshima T, Miyao J, et al. Identification and quantitative determination of fentanyl metabolites in patients by gas chromatography-mass spectrometry. Anesthesiology. 1984;61(1):73–7.
Mahlke NS, Ziesenitz V, Mikus G, Skopp G. Quantitative low-volume assay for simultaneous determination of fentanyl, norfentanyl, and minor metabolites in human plasma and urine by liquid chromatography-tandem mass spectrometry (LC-MS/MS). International journal of legal medicine. 2014;128(5):771–8. doi:10.1007/s00414-014-1040-y.
Higashikawa Y, Suzuki S. Studies on 1-(2-phenethyl)-4-(N-propionylanilino) piperidine (fentanyl) and its related compounds: novel metabolites in rat urine following injection of & alpha-methylfentanyl, one of the most abused typical designer drugs. Journal of Health Science. 2008;54(6):629–37. doi:10.1248/jhs.54.629.
Meyer MR, Dinger J, Schwaninger AE, Wissenbach DK, Zapp J, Fritschi G, et al. Qualitative studies on the metabolism and the toxicological detection of the fentanyl-derived designer drugs 3-methylfentanyl and isofentanyl in rats using liquid chromatography-linear ion trap-mass spectrometry (LC-MSn). Anal Bioanal Chem. 2012;402(3):1249–55. doi:10.1007/s00216-011-5528-8.
Staeheli SN, Baumgartner MR, Gauthier S, Gascho D, Jarmer J, Kraemer T, et al. Time-dependent postmortem redistribution of butyrfentanyl and its metabolites in blood and alternative matrices in a case of butyrfentanyl intoxication. Forensic Sci Int. 2016;266:170–7. doi:10.1016/j.forsciint.2016.05.034.
Patton AL, Seely KA, Pulla S, Rusch NJ, Moran CL, Fantegrossi WE, et al. Quantitative measurement of acetyl fentanyl and acetyl norfentanyl in human urine by LC-MS/MS. Anal Chem. 2014;86(3):1760–6. doi:10.1021/ac4036197.
Melent’ev AB, Kataev SS, Dvorskaya ON. Identification and analytical properties of acetyl fentanyl metabolites. J Anal Chem. 2015;70(2):240–8. doi:10.1134/s1061934815020124.
Wohlfarth A, Scheidweiler KB, Pang S, Zhu M, Castaneto M, Kronstrand R, et al. Metabolic characterization of AH-7921, a synthetic opioid designer drug: in vitro metabolic stability assessment and metabolite identification, evaluation of in silico prediction, and in vivo confirmation. Drug Test Anal. 2016;8(8):779–91. doi:10.1002/dta.1856.
Castaneto MS, Wohlfarth A, Desrosiers NA, Hartman RL, Gorelick DA, Huestis MA. Synthetic cannabinoids pharmacokinetics and detection methods in biological matrices. Drug Metab Rev. 2015;47(2):124–74. doi:10.3109/03602532.2015.1029635.
Roman M, Strom L, Tell H, Josefsson M. Liquid chromatography/time-of-flight mass spectrometry analysis of postmortem blood samples for targeted toxicological screening. Anal Bioanal Chem. 2013;405(12):4107–25. doi:10.1007/s00216-013-6798-0.
Holcapek M, Jirasko R, Lisa M. Basic rules for the interpretation of atmospheric pressure ionization mass spectra of small molecules. J Chromatogr A. 2010;1217(25):3908–21. doi:10.1016/j.chroma.2010.02.049.
Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacological Reviews. 1975;27(2):135–206.
Meyer MR, Maurer HH. Enantioselectivity in the methylation of the catecholic phase I metabolites of methylenedioxy designer drugs and their capability to inhibit catechol-O-methyltransferase-catalyzed dopamine 3-methylation. Chem Res Toxicol. 2009;22(6):1205–11. doi:10.1021/tx900134e.
Testa B, Mayer JM. The hydration of epoxides. Hydrolysis in drug and prodrug metabolism. Verlag Helv Chim Acta. 2006; p. 591–661.
Nebert DW, Roe AL, Vandale SE, Bingham E, Oakley GG. NAD(P)H:quinone oxidoreductase (NQO1) polymorphism, exposure to benzene, and predisposition to disease: a HuGE review. Genet Med. 2002;4(2):62–70.
Sobolevsky T, Prasolov I, Rodchenkov G. Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test Anal. 2012;4(10):745–53. doi:10.1002/dta.1418.
Wohlfarth A, Pang S, Zhu M, Gandhi AS, Scheidweiler KB, Liu HF, et al. First metabolic profile of XLR-11, a novel synthetic cannabinoid, obtained by using human hepatocytes and high-resolution mass spectrometry. Clin Chem. 2013;59(11):1638–48. doi:10.1373/clinchem.2013.209965.
Feasel MG, Wohlfarth A, Nilles JM, Pang S, Kristovich RL, Huestis MA. Metabolism of carfentanil, an ultra-potent opioid, in human liver microsomes and human hepatocytes by high-resolution mass spectrometry. AAPS J. 2016:1–11. doi:10.1208/s12248-016-9963-5.
Cashman JR, Park SB, Yang ZC, Wrighton SA, Jacob P, Benowitz NL. Metabolism of nicotine by human liver microsomes: stereoselective formation of trans-nicotine N′-oxide. Chem Res Toxicol. 1992;5(5):639–46. doi:10.1021/tx00029a008.
Pirmohamed M, Williams D, Madden S, Templeton E, Park BK. Metabolism and bioactivation of clozapine by human liver in vitro. J Pharmacol Exp Ther. 1995;272(3):984–90.
Peterson LA. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem Res Toxicol. 2013;26(1):6–25. doi:10.1021/tx3003824.
Kalgutkar AS, Gardner I, Obach RS, Shaffer CL, Callegari E, Henne KR, et al. A comprehensive listing of bioactivation pathways of organic functional groups. Current Drug Metabolism. 2005;6(3):161–225. doi:10.2174/1389200054021799.
Diao X, Wohlfarth A, Pang S, Scheidweiler KB, Huestis MA. High-resolution mass spectrometry for characterizing the metabolism of synthetic cannabinoid THJ-018 and its 5-fluoro analog THJ-2201 after incubation in human hepatocytes. Clin Chem. 2016;62(1):157–69. doi:10.1373/clinchem.2015.243535.
Steuer AE, Williner E, Staeheli S, Kraemer T. Studies on the metabolism of the fentanyl-derived designer drug butyrfentanyl in human in vitro liver preparations and authentic human samples using liquid chromatography-high resolution mass spectrometry (LC-HRMS). Drug Test Anal. 2016:(in press). doi:10.1002/dta.2111.
Acknowledgements
The authors thank Advanced Chemistry Development (ACD/Labs) for providing MetaSense™ software as well as Richard Lee and Edward Milton for their help. This research was conducted within the Strategic Research Area Forensic Sciences (Strategiområdet Forensiska Vetenskaper) at Linköping University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplementary Fig. 1
MSMS spectra and proposed fragmentation pattern of acetylfentanyl and its major metabolites in hydrolyzed human urine samples. (PDF 63 kb)
Supplementary Fig. 2
MSMS spectra and proposed fragmentation pattern of acrylfentanyl and its major metabolites in hydrolyzed human urine samples. (PDF 108 kb)
Supplementary Fig. 3
MSMS spectra and proposed fragmentation pattern of 4-fluoroisobutyryllfentanyl and its major metabolites in hydrolyzed human urine samples. (PDF 111 kb)
Supplementary Fig. 4
MSMS spectra and proposed fragmentation pattern of furanylfentanyl and its major metabolites in hydrolyzed human urine samples. (PDF 94 kb)
Rights and permissions
About this article
Cite this article
Watanabe, S., Vikingsson, S., Roman, M. et al. In Vitro and In Vivo Metabolite Identification Studies for the New Synthetic Opioids Acetylfentanyl, Acrylfentanyl, Furanylfentanyl, and 4-Fluoro-Isobutyrylfentanyl. AAPS J 19, 1102–1122 (2017). https://doi.org/10.1208/s12248-017-0070-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0070-z