ABSTRACT
For sugars to act as successful stabilizers of proteins during lyophilization and subsequent storage, they need to have several characteristics. One of them is that they need to be able to form interactions with the protein and for that miscibility is essential. To evaluate the influence of protein-sugar miscibility on protein storage stability, model protein IgG was lyophilized in the presence of various sugars of different molecular weight. By comparing solid-state nuclear magnetic resonance spectroscopy relaxation times of both protein and sugar on two different timescales, i.e., 1H T1 and 1H T1ρ, miscibility of the two components was established on a 2–5- and a 20–50-nm length scale, respectively, and related to protein storage stability. Smaller sugars showed better miscibility with IgG, and the tendency of IgG to aggregate during storage was lower for smaller sugars. The largest sugar performed worst and was phase separated on both length scales. Additionally, shorter protein 1H T1 relaxation times correlated with higher aggregation rates during storage. The enzyme-linked immunosorbent assay (ELISA) assay showed overlapping effects of aggregation and chemical degradation and did not correspond as well with the miscibility. Because of the small scale at which miscibility was determined (2–5 nm) and the size of the protein domains (∼2.5 × 2.5 × 5 nm), the miscibility data give an indirect measure of interaction between protein and sugar. This reduced interaction could be the result of steric hindrance, providing a possible explanation as to why smaller sugars show better miscibility and storage stability with the protein.
Similar content being viewed by others
INTRODUCTION
Protein pharmaceuticals have become increasingly important in the pharmaceutical industry over the past decade, with 10 out of the 41 drugs approved by the American Food and Drug Administration (FDA) in 2014 being proteins (1). Most protein formulations are produced as solutions requiring refrigerated storage and handling, the so-called cold chain. This is both costly and impractical. The cold chain can be circumvented by drying the protein formulation in the presence of a stabilizer, using methods such as lyophilization and spray drying (2, 3). For these situations, sugars are often used as stabilizers.
Much research has been done into the mechanism by which these sugars stabilize proteins both during drying and storage (4–6), producing several widely discussed theories regarding these mechanisms (e.g., vitrification theory, the water replacement theory, and refinements focusing on local mobility of the protein) (5, 7). For each of those theories, interactions between protein and sugar are needed, and for that, miscibility of the protein and sugar is a necessary condition.
To qualify solids as miscible or immiscible, it is important to define the scale of this miscibility and the technique used to quantify miscibility. Two substances can appear miscible using a technique that measures a larger domain size, while showing phase separation using a technique that measures a smaller domain size. Therefore, a definition of miscibility should be accompanied by a qualification of the scale at which it was established. In this manuscript, miscibility was determined using solid-state nuclear magnetic resonance spectroscopy (ssNMR). The method uses the concept of spin diffusion to determine the homogeneity of mixed systems (8). In homogeneous systems, spin diffusion causes magnetization transfer between neighboring nuclei, resulting in similar relaxation times for both nuclei. For phase separated systems, magnetization transfer does not occur and the relaxation times of different components are not averaged.
Spin diffusion is a time-dependent phenomenon and the length scale of spin diffusion L is correlated to the relaxation time as follows:
D is the spin diffusion coefficient, which is typically assumed to be 10−12 cm2/s (9, 10) and t is the relaxation time. When relaxation times of different timescales are compared, this principle allows differentiation of homogeneity on different length scales. In particular, a spin-lattice relaxation time (1H T1) is typically 1–5 s, corresponding to a spin diffusion over a distance of approximately 20–50 nm, while a rotating frame spin-lattice relaxation time (1H T1ρ) is usually 5–50 ms during which spin diffusion occurs over a distance of around 2–5 nm.
By comparing 1H T1 and 1H T1ρ relaxation times of different components in a mixture, in this case protein and sugar, it is thus possible to establish the intimacy of mixing on two different length scales (Fig. 1). If a system is fully miscible, both the relaxation times will be similar for both components. If a system is phase separated, both 1H T1 and 1H T1ρ values will be different for the sugar and protein. When the 1H T1ρ values are different, but the 1H T1 values are similar, this indicates phase separation on the 2–5-nm scale but miscibility on the 20–50-nm scale (partial phase separation). Using these relaxation times, it is thus possible to determine miscibility of these systems on two different length scales.
Illustration of the effect of spin diffusion on measured area for three different degrees of miscibility. Modified with permission from Yuan et al. (8). Copyright 2015 American Chemical Society
Recently, it was shown that larger and molecularly more rigid sugars (e.g., oligo- and polysaccharides with a backbone through the sugar ring) are less efficient stabilizers of proteins than smaller sugars (e.g., disaccharides) (11). It was found that these larger sugars form fewer hydrogen bonds with the protein during the last part of lyophilization (12). This lack of interactions could be responsible for phase separation in these protein-sugar mixtures. Therefore, the influence of protein-sugar miscibility on protein stability will be investigated in this manuscript.
MATERIALS AND METHODS
Materials
Immunoglobulin G (IgG, from bovine serum) was obtained from Sigma-Aldrich, St. Louis, MO, USA. Trehalose was acquired from Ferro Pfanstiehl (Waukegan, IL, USA) and dextrans 1.5, 5, and 70 kDa from Pharmacosmos (Holbaek, Denmark). Inulins 1.8 and 4 kDa were generous gifts from Sensus (Roosendaal, The Netherlands). Sodium phosphate buffer components used for the formulations and dialysis (monobasic as monohydrate, dibasic as heptahydrate) were purchased at Fisher Scientific.
Formulation Preparation
IgG was received frozen and thawed slowly on ice prior to use. The protein solution (IgG) was dialyzed at least three times for a minimum of 3 h against 2 mM sodium phosphate buffer (pH 7.5, SPB) at 4°C using a Spectra/Por® 6 dialysis tube membrane with a molecular weight cutoff of 2000 Da. After dialysis, the protein solution was filtered using Millex-GV low-protein binding PVDF syringe filters with a 0.22-μm pore size and 33-mm diameter. Subsequently, protein concentration was determined spectroscopically at 280 nm, using absorption coefficients A 1 %1 cm = 14 (13). The protein stock solution concentration was adjusted to 10.71 mg/mL by dilution with filtered SPB. Sugar stock solutions were prepared by dissolving sugars in SPB to a concentration of 100 mg/mL. If needed, solutions were heated to dissolve the sugar. All formulations were also filtered using 0.22 μM PVDF filters. Protein and sugar solutions were mixed to achieve an end concentration of 6 mg/mL protein and 44 mg/mL sugar (12% protein on dry solids basis). When IgG and dextran 70 kDa were mixed, some hazing was observed in the resulting solution. The solutions were transferred to Schott Fiolax® tubular vials of 10 mL with a 13-mm opening. For samples for physicochemical testing, a fill volume of 5 mL was used, and for stability testing, a fill volume of 1 mL was used.
Lyophilization
Freeze-drying was conducted using a SP Scientific LyoStar 3 (Warminster, PA, USA) equipped with Praxair ControLyo™ controlled ice nucleation technology. Compared to slow freezing, controlled nucleation is expected to reduce the risk of phase separation during freezing by saturation of either of the components and increase sample homogeneity. Controlled nucleation is thus expected not to have a negative impact on miscibility. Samples were placed on a shelf pre-cooled at 5°C and kept at that temperature for 30 min. The temperature was then lowered to −5°C at a rate of 1°C/min and held at that temperature for 30 min. Controlled nucleation was then induced using argon as a carrier gas, followed by further cooling to −40°C at a rate of 1°C/min. After 60 min at that temperature, the pressure was lowered to 87 μbar and the temperature was raised to −25°C at 1°C/min. Sample temperature of at least five vials was monitored using thermocouples. Shelf temperature was lowered to −28°C if primary drying was not done after 30 h or if the product temperature would exceed −33°C. The end of primary drying was established by comparative pressure measurement (i.e., Pirani vs. MKS Baratron) (14). After primary drying was completed, the shelf temperature was slowly raised to 40°C at a rate of 0.1°C/min and secondary drying was continued for 6 h at this temperature. Lastly, the vials were stoppered in the freeze dryer at 87 μbar using Flurotec™ stoppers and were sealed with aluminum crimpers upon removal from the freeze dryer. All samples were stored at −20°C up until the moment of analysis. Water content of the lyophilizates was determined using Karl Fisher coulometry and was found to be ≤0.25% for all tested formulations.
Solid-State Nuclear Magnetic Resonance Spectroscopy
All solid-state NMR spectra were acquired using a Tecmag Redstone HF3 (Tecmag, Houston, TX) spectrometer operating at 100.6 MHz for 13C (9.4 T static magnetic field). The lyophilized powders were packed into 7-mm zirconia rotors and sealed with Kel-F end-caps (Revolution NMR, LLC, Fort Collins, CO) in a glovebox purged with dry nitrogen gas with a relative humidity of <1%. Experiments were performed using a 7-mm double resonance magic angle spinning (MAS) probe (Agilent, Palo Alto, CA). All 13C spectra were acquired under MAS (15) at 4 kHz, using ramped-amplitude cross-polarization (CP) (16), total sideband suppression (TOSS) (17), and spinal64 decoupling (18) with a 1H decoupling field of about 66 kHz. A 2-ms contact time was used in all experiments. 3-Methylglutaric acid was used to optimize spectrometer settings, and the methyl peak was referenced to 18.84 ppm (19). All spectra were acquired at ambient temperature.
Determination of Phase Separation
To evaluate phase separation of the protein and sugar, the 1H T1 and 1H T1ρ values were determined. The 1H T1 relaxation values were measured using a saturation recovery experiment through 13C observation. The peak areas were integrated and plotted in KaleidaGraph (Synergy Software, Reading, PA) and fitted to the following equation to determine 1H T1 times for the protein and the sugar peaks individually:
where M is the integrated signal intensity, M 0 is an amplitude parameter from the fit, τ is the recovery delay time, and 1H T 1 is the obtained spin-lattice relaxation time. The protein peak was integrated in the ∼164–180-ppm region, whereas for the sugar peak, the ∼90–106-ppm region was used. Only the central one third of the peaks was used. These peaks were chosen because of the lack of overlap between the two species in those areas. For the 1H T1ρ experiment, the spin locking time was varied from 1 to 30 ms. Similar to the 1H T1 experiments, Kaleidograph was used for integration and fitting of the data using formula 3.
The symbols are the same as used in formula 2. 1H T1p is the rotating frame spin-lattice relaxation time.
Data acquisition parameters for the 1H T1 experiments were a 1-s recycle delay, 2048 acquisition points (51.2 ms acquisition time), a 3.8-μs 1H 90° pulse (∼66 kHz decoupling field), a 2-ms contact time, and a 4-kHz MAS frequency. Twelve time points (0.1, 0.3, 1.2, 1.8, 3.0, 4.0, 9.0, and 15 s) were used in the 1H T1 determination, and 500 acquisitions were acquired per T1 point. Data acquisition parameters for the 1H T1rho experiments were a 4-s recycle delay, 2048 acquisition points (51.2 ms acquisition time), a 3.8-μs 1H 90° pulse (∼66 kHz decoupling field), a 2-ms contact time, and a 4-kHz MAS frequency. Eight spin locking times (1, 2, 3, 5, 10, 15, 20, and 30 ms) were used, with 500 acquisitions per T1 point. Data acquisition for the 13C spectra was a 4-s recycle delay, a 2-ms cross-polarization contact time, 2048 acquisition points (51.2 ms acquisition time), and 1000 acquisitions.
Data processing was done the same way for all spectra and was done in the TNMR software package (Tecmag, Houston, TX). The first 512 points (13.3 ms acquisition time) were used and Fourier transformed with 20 Hz of line broadening. The spectra (supplemental 1) were individually phased and integrated using abovementioned limits and the fit to the equations above. The central one third of the peaks was used for the integration to obtain the best data and minimize error of the peak integrations. The error presented for the peaks is the error of the fit from the KaleidaGraph fitting. Protein and sugar relaxation times were considered different if they were more than two fitting errors apart.
Storage Stability
To evaluate the stabilizing effects of the different sugars, the different formulations were subjected to an accelerated storage stability test. Samples were stored at 60°C up to 90 days. Samples were taken from the freezer at different time points, so they could all be analyzed together, eliminating assay variations. The storage temperature was below the glass transition temperature (Tg) of all samples tested, and they therefore remained physically as original during storage.
High Pressure Size Exclusion Chromatography (HPSEC)
HPSEC was conducted to provide information on aggregation of the IgG. The samples which were reconstituted with 1 mL ultrapure water were diluted further to a concentration of 1 mg/mL IgG with 20 mM NaH2PO4 with 150 mM NaCl (pH 7.5). The samples were filtered using a 0.45-μm filter before being injected on a Superose 6 GL 10/30 column in 100-μL aliquots. The mobile phase was the same as the dilution buffer, and the flow used was 0.5 mL/min. Detection was done spectrophotometrically at 214 nm.
Baseline corrections based on the signals between 6 and 7 and 26–27 min were carried out for the obtained chromatograms. The area under the curve of the monomer peak (∼16 min) was determined from half height to half height of the peak. Degradation kinetics were established by correlating monomer peak area and the square root of storage time and carrying out a linear regression analysis; the slope of that curve was used as a measure for the aggregation rate. To limit aggregation after reconstitution of the samples, each sample was measured only once.
Enzyme-Linked Immunosorbent Assay (ELISA)
Since HPSEC only provides information about the aggregation of IgG but does not give information about functionality, an ELISA was conducted on the same samples to provide additional information. The integrity of the epitope of IgG was used as a measure for its stability. This was established using a bovine IgG ELISA kit supplied by Bethyl (Montgomery, TX, USA). Lyophilized samples were reconstituted using 1 mL of ultrapure water and diluted to concentrations between 500 and 7.8 ng/mL using diluent buffer, which consisted of 50 mM Tris with 0.14 M NaCl and 0.05% Tween 20 (pH 8.0). Analysis was carried out on 96-well plates, which were pre-incubated with affinity-purified bovine IgG coating antibody for an hour at room temperature and subsequently blocked with blocking solution, using the diluent buffer, at the same temperature for 30 min. The diluted samples were incubated for an hour at room temperature, followed by incubation of horseradish peroxidase-conjugated bovine IgG detection antibody for an hour. Lastly, a substrate solution containing 3,3′,5,5′-tetramethybenzidine (TMB) was added and incubated in the dark during 15 min at room temperature. The reaction was stopped by addition of 0.18 M H2SO4, after which the conversion of TMB by peroxidase was measured spectrophotometrically at 450 nm. The plates were washed five times with diluent buffer between all steps up to the addition of TMB. The absorbance was related to an unprocessed reference sample, giving an indication of the amount of epitopes still intact. Results were not related to t = 0 as some formulations already showed loss of epitopes during lyophilization. Each sample was measured in four dilutions, and results from the two optimal dilutions were averaged. A duplicate analysis of a large part of the formulations provided nearly identical results.
RESULTS
Solid-State Nuclear Magnetic Resonance Spectroscopy
For the formulations of trehalose, dextran 1.5 kDa, and both inulins, the 1H T1 relaxation times of protein and sugar are indistinguishable (Fig. 2a). For dextran 5 and 70 kDa, the values of the protein and sugar are clearly different, with a bigger difference in 1H T1 relaxation times for the formulation of dextran 70 kDa. This is indicative of phase separation at the 20–50-nm scale for formulations with dextran 5 and 70 kDa and IgG. For the formulations with IgG and trehalose, both inulins, and dextran 1.5 kDa, there is no indication of phase separation on this scale.
In addition to phase separation, there is a trend in the 1H T1 relaxation time, where the lower molecular weight sugar systems had the longest relaxation time, and the protein relaxation time decreases as the sugar molecular weight increases. Since 1H T1 relaxation times are indicators of molecular mobility, the longer relaxation times would correlate with a lower molecular mobility (20, 21). The absolute 1H T1 relaxation time of IgG is highest for the smallest sugar trehalose and decreases when the molecular weight of the combined sugar increases, suggesting less molecular mobility of the protein when incorporated in the low molecular weight sugar.
The 1H T1ρ relaxation times of IgG do not show an increase with lower molecular weight (Fig. 2b). 1H T1ρ relaxation times are indistinguishable for trehalose and IgG, somewhat different for the inulin formulations, and even more different for the formulations containing dextran. This indicates miscibility down to the 2–5-nm scale for trehalose. The other formulations show immiscibility on this scale. Therefore, combinations of IgG with both inulins and dextran 1.5 kDa are classified as partially phase separated, whereas dextrans 5 and 70 kDa are phase separated and trehalose and IgG are fully miscible.
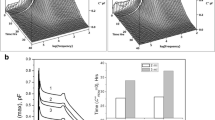
Storage Stability—HPSEC
During storage at 60°C, the formulation containing trehalose shows nearly no aggregation of IgG (Fig. 3). For the other sugars, there is an increasing degree of IgG aggregation with increasing molecular weight of the sugar, with the exception of dextran 5 kDa. The formulation with dextran 1.5 kDa shows ∼20% of aggregation of IgG after 90 days of storage, and for inulin 1.8 kDa and dextran 5 kDa, this is ∼40%. Inulin 4 kDa shows ∼50% loss of monomer, and lastly there was no clear difference in aggregation between the formulation without sugar and that with dextran 70 kDa. The aggregation results correlate with the 1H T1 relaxation times of the protein and are consistent with the more stable samples being fully or partially miscible and the phase separated samples having the poorest stability.
Storage Stability—ELISA
As with aggregation, the formulation of IgG and trehalose shows little or no change in amount of intact epitopes during storage (Fig. 4). Inulin 4 kDa shows a loss of around 30% of epitopes, and the formulations with dextran 70 kDa and the formulation without sugar lost ∼40% of epitopes. Remarkably, the smaller inulin 1.8 kDa and the two smaller dextrans 1.5 and 5 kDa show a bigger loss of intact epitopes than the formulation without sugar, a total loss of ∼50%, ∼70%, and ∼60%, respectively. This indicates a deteriorative effect of the sugar on the protein, which could indicate that the reducing groups of the sugar reacted with the amine groups of the protein, the so-called Maillard reaction. Initial Maillard browning could affect the functional epitopes of IgG and therewith the ELISA data, but because of the molecular weight of these sugars compared to that of IgG, it would likely not impact HPSEC results significantly. Some discoloration was observed in the samples with dextran 1.5 kDa, dextran 5 kDa, and inulin 1.5 kDa after 90 days. Most discoloration was found in the formulation containing dextran 1.5 kDa. There was no observable discoloration for formulations with trehalose, inulin 4 kDa and dextran 70 kDa. It should be noted, however, that other chemical degradation reactions could not be ruled out.
DISCUSSION
In this study, the influence of miscibility of protein-sugar lyophilizates on the storage stability was investigated using model protein IgG. Miscibility was evaluated by comparing ssNMR relaxation times at two timescales, providing information on miscibility on 2–5- and 20–50-nm length scales. When 1H T1 relaxation times of protein and sugar were different for a single sample, this indicated immiscibility of the system already on the larger 20–50-nm scale and these combinations were classified as immiscible. When 1H T1 relaxation times were similar, but 1H T1ρ relaxation times, with a diffusion length scale of 2–5 nm, were different, the combination was considered partially phase separated. If both 1H T1 and 1H T1ρ relaxation times were similar, there was no indication of phase separation and the sample was deemed miscible. Table I shows the classifications of all the tested combinations, based on this rationale.
Previously, we reported that smaller and molecularly more flexible sugars were better capable of stabilizing proteins during lyophilization (11), as they were less inhibited by steric hindrance and configurational inflexibility and therewith better able to form hydrogen bonds with the protein during lyophilization (12). Here, it was found that miscibility and absolute 1H T1 relaxation times decreased with increasing molecular weight of the sugar used. 1H T1 relaxation times have been shown to be predictive for physical stability of amorphous systems (22) and protein storage stability (20), presumably because the molecular mobility involved in generating the 1H T1 relaxation process is similar to the molecular mobility that causes degradation. Here too, protein 1H T1 relaxation times correlate with protein aggregation rates (Fig. 5).
The overall size of IgG is significantly larger than 2–5 nm, with a total maximum length of ∼16 nm (23). However, the protein is not a sphere and its subdomains (chains) are smaller, with dimensions of approximately 2.5 × 2.5 × 5 nm (24). Therefore, these miscibility data likely describe the ability of sugars to enter empty spaces in the protein’s three-dimensional structure and form intimate contact. Thus, the miscibility we describe here could possibly indirectly indicate steric hindrance of the protein and sugar. This is in line with our previous findings, as a close proximity of the sugar to the protein is prerequisite for hydrogen bonding and therewith stabilization. It is therefore not surprising that the smallest sugar tested, disaccharide trehalose, is the best stabilizer of IgG as represented by both the HPSEC and ELISA storage stability data and that stabilizing capacity diminishes with increasing molecular weight of the stabilizer.
The ELISA data do not correlate as well with the miscibility data as the HPSEC results do. Most likely, this is because of an additional mechanism of degradation, presumably the Maillard reaction or other chemical degradation reactions. Inulin without the glucose end group and dextran both have a reducing end group, which is relatively more abundant in lower molecular weight sugars (25). This would explain why the smaller dextrans (1.5 and 5 kDa) and smaller inulin (1.8 kDa) maintain less functional epitopes than the protein without sugar. As mentioned previously, chemical bonding of a sugar of 1.5–5 kDa, as happens in the initial steps of the Maillard cascade, does not have a large impact on the total mass and three-dimensional size of IgG, which has a molecular weight of approximately 150 kDa. Therefore, HPSEC is insensitive to Maillard reactions with relatively small oligosaccharides, unlike the ELISA assay which will detect these changes if they impact the functional epitope directly or indirectly do so by causing conformational changes.
In this study, a sugar-to-protein weight ratio of approximately 7:1 was used, which is on the low end of ratio’s commonly used in protein-sugar lyophilizates (26). The sugar-to-protein ratio most likely influences miscibility. As the relative amount of sugar increases, saturation of the microenvironment of the protein can occur, resulting in phase separation. This was not observed here, as the formulation with trehalose was found to be miscible. However, using this concept, it should be possible to establish at which sugar-protein ratio saturation occurs by measuring different sugar-protein ratios of one formulation. Knowing at which point saturation occurs could be useful information for formulation development.
Considering the above, smaller non-reducing sugars are preferred for stabilizing proteins as they are less limited by steric hindrance and are thus more miscible with the protein. However, these sugars are generally characterized by a lower Tg, and with that, care should be taken that vitrification of the formulation is maintained during storage (27). That is, one should not exceed the glass transition temperature. This is particularly a problem in case of higher levels of residual moisture or moisture sorption, as water has a strong plasticizing effect on sugar glasses (28). In cases where the Tg would not be adequate for the planned storage conditions (i.e., approximately 10–20°C higher than the storage temperature (29)), combining a large polysaccharide with a non-reducing disaccharide is a suitable alternative (30, 31). This limits steric hindrance as much as possible, while achieving a high enough Tg (11).
CONCLUSION
In summary, ssNMR is able to detect miscibility on a level close to the size of the protein molecules and the obtained miscibility classifications and 1T H1 relaxation times correlate well with aggregation rates of IgG during storage. The ELISA storage stability data show degradation by both aggregation and Maillard browning or other chemical degradation reactions, which cannot be deconvoluted accurately. Smaller sugars show better protein-sugar miscibility as they are less influenced by steric hindrance, and this makes them more suitable as stabilizers of proteins.
REFERENCES
U.S. Food and Drug Administration Center for Drug Evaluation and Research. Novel new drugs 2014 summary [Internet]. FDA. 2015. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/UCM430299.pdf
Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14(8):969–75.
Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203(1–2):1–60.
Chang L, Shepherd D, Sun J, Ouellette D, Grant KL, Tang X, et al. Mechanism of protein stabilization by sugars during freeze-drying and storage: native structure preservation, specific interaction, and/or immobilization in a glassy matrix? J Pharm Sci. 2005;94(7):1427–44.
Chang L, Pikal M. Mechanisms of protein stabilization in the solid state. J Pharm Sci. 2009;98(9):2886–908.
Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27(4):544–75.
Yoshioka S, Aso Y. Correlations between molecular mobility and chemical stability during storage of amorphous pharmaceuticals. J Pharm Sci. 2007;96(5):960–81.
Yuan X, Sperger D, Munson EJ. Investigating miscibility and molecular mobility of nifedipine-PVP amorphous solid dispersions using solid-state NMR spectroscopy. Mol Pharm. 2014;11(1):329–37.
Bovey FA, Mirau PA. NMR of polymers. 1st ed. San Diego: Academic; 1996.
Aso Y, Yoshioka S, Miyazaki T, Kawanishi T, Tanaka K, Kitamura S, et al. Miscibility of nifedipine and hydrophilic polymers as measured by (1)H-NMR spin-lattice relaxation. Chem Pharm Bull (Tokyo). 2007;55(8):1227–31.
Tonnis WF, Mensink MA, de Jager A, van der Voort Maarschalk K, Frijlink HW, Hinrichs WLJ. Size and molecular flexibility of sugars determine the storage stability of freeze-dried proteins. Mol Pharm. 2015;12(3):684–94.
Mensink MA, Van Bockstal P-J, Pieters S, De Meyer L, Frijlink HW, van der Voort Maarschalk K, et al. In-line near infrared spectroscopy during freeze-drying as a tool to measure efficiency of hydrogen bond formation between protein and sugar, predictive of protein storage stability. Int J Pharm. 2015;496(2):792–800.
Sigma-Aldrich. Product information IgG from bovine serum [Internet]. 2015 [cited 2015 Dec 27]. Available from: http://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/2/i9640pis.pdf
Patel SM, Doen T, Pikal MJ. Determination of end point of primary drying in freeze-drying process control. AAPS PharmSciTech. 2010;11(1):73–84.
Andrew ER, Bradbury A, Eades RG. Removal of dipolar broadening of nuclear magnetic resonance spectra of solids by specimen rotation. Nature. 1959;183(4678):1802–3.
Pines A, Gibby M, Waugh J. Proton-enhanced NMR of dilute spins in solids. J Chem Phys. 1973;59(2):569.
Dixon W, Schaefer J, Sefcik M, Stejskal E, McKay R. Total suppression of sidebands in CPMAS C-13 NMR. J Magn Reson. 1982;49(2):341–5.
Fung BM, Khitrin AK, Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson. 2000;142(1):97–101.
Barich DH, Gorman EM, Zell MT, Munson EJ. 3-Methylglutaric acid as a 13C solid-state NMR standard. Solid State Nucl Magn Reson. 2006;30(3–4):125–9.
Lam Y-H, Bustami R, Phan T, Chan H-K, Separovic F. A solid-state NMR study of protein mobility in lyophilized protein-sugar powders. J Pharm Sci. 2002;91(4):943–51.
Yoshioka S, Aso Y, Kojima S. Different molecular motions in lyophilized protein formulations as determined by laboratory and rotating frame spin-lattice relaxation times. J Pharm Sci. 2002;91(10):2203–10.
Dempah KE, Barich DH, Kaushal AM, Zong Z, Desai SD, Suryanarayanan R, et al. Investigating gabapentin polymorphism using solid-state NMR spectroscopy. AAPS PharmSciTech. 2013;14(1):19–28.
Harris LJ, Larson SB, Hasel KW, McPherson A. Refined structure of an intact IgG2a monoclonal antibody. Biochemistry. 1997;36(7):1581–97.
Reth M. Matching cellular dimensions with molecular sizes. Nat Immunol. 2013;14(8):765–7.
Mensink MA, Frijlink HW, van der Voort Maarschalk K, Hinrichs WLJ. Inulin, a flexible oligosaccharide I: review of its physicochemical characteristics. Carbohydr Polym. 2015;130:405–19.
Mensink MA, Frijlink HW, Vander Voort Maarschalk K, Hinrichs WLJ. Inulin, a flexible oligosaccharide II: review of its pharmaceutical applications. Carbohydr Polym. 2015;134:418–28. Elsevier Ltd.
Crowe JH, Carpenter JF, Crowe LM. The role of vitrification in anhydrobiosis. Annu Rev Physiol. 1998;60(1):73–103.
Roos Y, Karel M. Plasticizing effect of water on thermal behavior and crystallization of amorphous food models. J Food Sci. 1991;56(1):38–43.
Grasmeijer N, Stankovic M, de Waard H, Frijlink HW, Hinrichs WLJ. Unraveling protein stabilization mechanisms: vitrification and water replacement in a glass transition temperature controlled system. Biochim Biophys Acta Proteins Proteomics. 2013;1834(4):763–9.
Allison SD, Manning MC, Randolph TW, Middleton K, Davis A, Carpenter JF. Optimization of storage stability of lyophilized actin using combinations of disaccharides and dextran. J Pharm Sci. 2000;89(2):199–214.
Garzon-Rodriguez W, Koval RL, Chongprasert S, Krishnan S, Randolph TW, Warne NW, et al. Optimizing storage stability of lyophilized recombinant human interleukin-11 with disaccharide/hydroxyethyl starch mixtures. J Pharm Sci. 2004;93(3):684–96.
ACKNOWLEDGMENTS
This research was jointly financed by Royal FrieslandCampina, the European Union, European Regional Development Fund and The Ministry of Economic Affairs, Agriculture and Innovation, Peaks in the Delta, the Municipality of Groningen, the Provinces of Groningen, Fryslân, and Drenthe as well as the Dutch Carbohydrate Competence Center.
We acknowledge Ms. de Ruiter and Mr. Scheffer of Royal FrieslandCampina for their analytical support.
MJN and EJM acknowledge support from the NSF I/UCRC Center for Pharmaceutical Development (IIP-1063879 and industrial contributions) for financial support. The solid-state NMR results presented here are from academic work performed at the University of Kentucky, and no data from Kansas Analytical Services are presented. EJM is a partial owner of Kansas Analytical Services, a company that provides solid-state NMR services to the pharmaceutical industry.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(GIF 70 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mensink, M.A., Nethercott, M.J., Hinrichs, W.L.J. et al. Influence of Miscibility of Protein-Sugar Lyophilizates on Their Storage Stability. AAPS J 18, 1225–1232 (2016). https://doi.org/10.1208/s12248-016-9937-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9937-7