Abstract
Background
Anthemis palestina (Asteraceae) extends across the Mediterranean region, southwest Asia and eastern Africa. Although traditionally used for several applications, in vitro investigation of biological functions associated with Anthemis palestina essential oil had never been reported.
Methods
The air-dried flowers of Anthemis palestina were subjected to hydrodistillation to yield the oil. The antioxidant activity of the hydrodistilled oil was characterized using various in vitro model systems such as DPPH, ferric-reducing antioxidant power and hydroxyl radical scavenging activity. Antibacterial activity was tested against six bacterial species, representing both Gram positive and Gram negative bacteria. Antifungal activity was evaluated using three Candida species. The minimum inhibitory concentration (MIC) for each examined microorganism was determined using the microdilution method. The oil’s antiproliferative effects against eight human cancer cell lines were also studied and the lethal doses that resulted in 50% reduction of cell viability (LD50) were determined.
Results
The results indicate that the essential oil of Anthemis palestina exhibited substantial antioxidant activities as demonstrated with DPPH, ferric reducing antioxidant power, and hydroxyl radical scavenging activity. In addition, a broad-spectrum antibacterial activity of the oil was revealed with better susceptibility of Gram positive bacteria towards the oil. The MIC values ranged between 6–75 μg/ml. Besides, the oil demonstrated a moderate inhibitory effect on the three Candida species examined; with MIC values ranging between 48–95 μg/ml. Potent cytotoxic activities, especially against HeLa cell line; with LD50 of 32 μg/ml, BJAB cell line; with LD50 of 57 μg/ml, and Caco-2 cell line; with LD50 of 61 μg/ml, were observed.
Conclusion
The results obtained indicate high potential of Anthemis palestina essential oil as bioactive oil, for nutraceutical and medical applications, possessing antioxidant, antimicrobial and antiproliferative activities.
Similar content being viewed by others
Background
The genus Anthemis is one of the most important genera of the Asteraceae family and comprises of approximately 210 species [1]. The geographic distribution of Anthemis extends across Europe, Southwestern Asia, Northern and Northeastern Africa, Southern Arabia, and tropical East Africa [2, 3]. Since the Roman times, the species of this genus have been commonly used in traditional medicine therapies [4, 5]. In the Mediterranean region, anti-inflammatory, antioxidant, antibacterial, and antispasmodic remedies are the most common traditional applications of the genus species.
During the last two decades, chemical composition of various extracts of Anthemis species was thoroughly investigated. Basically, flavonoids, polyphenolics and terpenes were reported as the main constituents of the plant [6, 7]. Recently, numerous Anthemis species have shown potential antimicrobial activity that could be correlated to their pehnolics and flavanoids composition [7, 8]. Additionally, digestive, antispasmodic and anti-Helicobacter pylori activities were all linked to Anthemis species in several reports [9–11]. On the other hand, the essential oils from different Anthemis species are frequently encountered as preservatives and flavoring agents in pharmaceuticals and cosmetic products [12–16].
Anthemis palestina Reut. ex Boiss., is distributed in the Middle and the Northern mountainous regions of Jordan, where it is known as Palestine chamomile due to resemblance to Roman and German chamomiles; the plant is an annual herb with flowering period between March-June [17]. Despite the extensive use of A. palestina in Folk medicine in Jordan, there have been only limited attempts to investigate the chemical or the biological properties of this plant in relation to its medicinal uses. Nonetheless, chemical composition of the essential oil isolated from flowers of A. palestina was recently reported by Tawaha et al.[18]. In the present study, in an effort to characterize the biological activities of A. palestina, we report here the antioxidant, antimicrobial, and antiproliferative activities associated with the essential oil isolated from the air-dried flowers of the plant. To our knowledge, thoroughly detailed studies on different aspects of the biological activity of A. palestina have not been reported yet.
Methods
Plant materials
The dried flowers of Anthemis palestina Reut. ex Boiss. were collected from Houfa region, Irbid (Northern Jordan), during its flowering phase (March to May), in Mid-April, 2012. The taxonomic identity of the collected plant was confirmed by Eng. Mohammad Al-Gharaibeh, a botanist, Department of Natural Resources and Environment, Faculty of Agriculture, Jordan University of Science and Technology (JUST), Irbid, Jordan. A voucher specimen (No. KT-AP-JOR-2012) has been deposited at the herbarium of the same institute.
Essential oil isolation
The air-dried flowering parts (whole flowers) of the collected plant were ground to about 0.5 mm particle size (30–35 mesh). To obtain the essential oil, 500 g of the ground plant material were accurately weighed and subjected to hydrodistillation using Clevenger-type apparatus for 4 hours. The hydrodistilled oil (yield ~ 0.7%) was dried over anhydrous sodium sulphate and reserved at 4°C in a sealed brown vial until required.
Essential oil composition analysis
Oil composition analysis was achieved via GC-FID and GC-MS analysis as reported in details in our previous In-Press work by Tawaha et al. [18]. Briefly, samples of the oil were injected to both system and analyzed under linear temperature programming. Compounds identification was performed by matching their spectra with the data bank mass spectra (WILEY, NIST and ADAMS-2007 libraries) and also by comparing their calculated arithmetic indices with reported values in the literature including the Adam’s library [18]. Identification of the main components was further confirmed by co-chromatography of their authentic standards under the same chromatographic conditions of samples analysis [18].
Antioxidant activity
DPPH free radical scavenging activity
The free-radical scavenging activity of the hydrodistilled oil was measured as a decrease in the absorbance of methanol solution of 2,2-diphenyl-1-picrylhydrazil (DPPH). A stock solution of DPPH (0.1 mM) was prepared in methanol and different concentrations of the essential oil were added (5–250 μg/ml). After incubation at room temperature for 30 min, the pale pink color developed was measured spectrophotometerically at 517 nm and compared with the standard (5–250 μg/ml ascorbic acid) [19]. Free radical scavenging activity was expressed as the percentage inhibition calculated using the following formula: % Anti-radical activity = (Control Absorbance- Sample Absorbance) × 100/Control Absorbance.
Hydroxyl radical scavenging activity
The oil (0.2 ml) at different concentrations (5–250 μg/ml), was added to 1 ml of iron-EDTA solution (0.13% ferrous ammonium sulphate and 0.26% EDTA), 0.5 ml of 0.018% EDTA, 1 ml of DMSO (0.85% v/v in 0.1 M phosphate buffer, pH 7.4) and 0.5 ml of 0.22% ascorbic acid were added to each tube. The tubes were capped tightly and heated in a water bath at 80-90°C for 15 min. The reaction was terminated by adding 1 ml of ice-cold trichloroacetic acid (TCA) (17.5% w/v). Afterward, 3 ml of Nash reagent (75.0 g of ammonium acetate, 3 ml of glacial acetic acid and 2 ml of acetyl acetone were mixed and distilled water was added to make up total volume of 1 L) was added to each tube, then left at room temperature for 15 min for color development. The intensity of the yellow color formed was measured at 412 nm against blank [20]. Percentage inhibition was determined by comparing the results of the test and the standard compound (ascorbic acid) by using the formula: % inhibition activity = (Control Absorbance- Sample Absorbance) × 100/Control Absorbance.
Ferric-reducing antioxidant power (FRAP) assay
The reducing power of the oil was determined by the Ferric-Reducing Antioxidant Power (FRAP) assay described by Yen and Chen [21]. One ml of different concentrations of the oil (5–250 μg/ml) was mixed with 2.5 ml of potassium phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide (1 g/100 ml). The mixture was incubated at 50°C for 20 minutes. TCA (10%, 2.5 ml) was added to the mixture to terminate the reaction. Finally, 2.5 ml of the supernatant solution was mixed with 2.5 ml distilled water and 0.5 ml ferric chloride. The procedure was repeated in triplicate and allowed to stand for 30 min before measuring the absorbance at 700 nm. Ascorbic acid was used as a positive control. The percentage of antioxidant activity in FRAP assay of the samples was calculated according to the formula:
Where,
A0 = Absorbance of the control (potassium phosphate buffer + FRAP reagent)
A1 = Absorbance of sample
Antimicrobial activity
Microorganisms
Six bacterial and three fungal species were obtained from the Microbial Culture Collection Center of Medicine School at The University of Jordan, Jordan. The species are: Bacillus subtilis ATCC 11562, Staphylococcus aureus ATCC 6538, Staphylococcus epidermidis ATCC 12228, Escherichia coli ATCC 29425, Pseudomonas aeuriginosa ATCC 11921, Xanthomonas vesicatoria ATCC 11633, Candida albicans ATCC 10231, Candida glabrata ATCC 1615, and Candida krusei ATCC 6258.
Determination of minimum inhibitory concentration (MIC)
The MICs of the oil against the microorganisms under investigation were assessed according to the broth microdilution method, as previously reported [22], with minor modifications. Briefly, 0.3 ml of the oil was dissolved in DMSO and serial diluted with the medium to the desired concentrations. MIC tests were carried out in 96 flat bottom microtiter plates (TPP, Switzerland). Each test well was filled with 100 μl nutrient broth. A sample (100 μl) of the stock solution was added to the first test well and mixed. A series of dilutions was then prepared across the plate. A 10 μl aliquot of the microorganism was used to inoculate each microtiter plate well to achieve a final inoculum size of 1 × 105 CFU/ml. Bacteria were grown in Mueller-Hinton broth (MHB; Oxoid, Basingstoke, UK) whereas Candida were grown in Yeast Peptone Dextrose (YPD) broth (BD Difco™). Positive controls, Norfloxacin for bacteria and Amphotericin B for fungi, and a negative control of the vehicle (DMSO), were prepared under the same experimental conditions and employed in triplicates.
Bacterial plates were incubated for 24 h at 37°C, whereas Candida plates were incubated for 48 h at 33°C, with shaking. After completion of the incubation period, optical densities were measured at 600 nm (OD600) using a Microplate Reader (Palo Alto, CA, USA). The minimum inhibitory concentration (MIC) value was defined as the lowest concentration that inhibited visible microbial growth of the tested microorganism. MIC determination was carried out in triplicates (in same 96-well plate) and repeated three times for each microorganism.
Antiproliferative activity
Cell culture
Cell lines were cultured in high glucose Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen, USA) containing 10% heat inactivated fetal bovine serum (HI-FBS) (Invitrogen), 2 mmol/l L-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. Cell lines were maintained at 37°C in a 5% CO2 atmosphere with 95% humidity. The cells were passaged weekly, and the culture medium was changed twice a week. The optimal plating densities were determined according to the cells growth profiles,
MTT assay
Cytotoxicity was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT, Sigma) assay, as previously described [23]. The concentration that inhibits the proliferation of oil-treated cells by 50% relative to the control (untreated cells) was used to evaluate the cytotoxic activity of the essential oil. To ensure exponential growth throughout the experimental period, as well as a linear relationship between absorbance at 570 nm and cell number when analyzed by the MTT assay, 2 × 104 cells per well were seeded of each cell line. Treated cells were incubated in a 37°C incubator with 5% CO2 for 48 h. As a positive control, vincristine sulphate (Sigma) was used at concentrations of 50 and 100 nM. In addition, a negative control; control wells without essential oils treatment, was employed and prepared under the same experimental conditions. All treatments were carried out in triplicates (in same 96-well plate) and repeated two times for each cell line.
Statistical analysis
All results were expressed as the mean ± standard deviation (SD). Data were analyzed with Statistical Package for Social Sciences program (SPSS) database for Windows, version 17 (SAS Institute, Cary, NC). The t-test was used to test for statistical significance with a p-value <0.05 deemed as significant.
Results and discussion
The simultaneous use of mass spectral and retention (Kovat’s) index matching allowed for the unequivocal identification of more than 97% of the components of the oil, obtained from flowers, as previously determined by the GC and GC-MS analyses [18]. The oil yield was 0.7% (v/w dried material). The analyses permitted the identification of about 109 compounds in the oil of A. palestina. The oil was characterized by dominant levels of sesquiterpenes (71.4%, hydrocarbons = 26.6% and oxygenated = 44.8%) and a moderate level of monoterpenes (19.4%), mainly oxygenated (18.5%). Spathulenol (9.8%) was the principal oil component, where, germacrene D (8.9%), caryophyllene oxide (6.8%), 3-thujanol acetate (3.7%), E-β-farnesene (3.5%), bornyl andelate (3.2%), 1,8-cineole (2.9%), salvial-4(14)en-1-one (2.5%), α-cadinol (2.4%), β-caryophyllene (2.3%) and some other components were the major oil constituents.
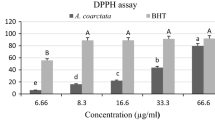
An antioxidant is defined as a substance that considerably suspends or inhibits an oxidation process [24]. The antioxidant activity is commonly determined via measuring the inhibition rate of an oxidation processes in the presence of an antioxidant [24]. Antioxidant efficiency is often linked to the antioxidant ability to scavenge stable free radicals. DPPH is extensively used to determine the antiradical activity of a given compound or extract. Figure 1 demonstrates the free radical scavenging activity of A. palestina essential oil relative to a standard antioxidant, such as ascorbic acid. As illustrated, the tested oil exhibits a dose-dependent scavenging activity against DPPH activity. In fact, the oil yielded percentage scavenging activities of 13%, 27%, 40% and 63% at concentrations of 10, 25, 50 and 100 μg/ml, respectively (Figure 1). Apparently, the oil of A. palestina possesses a moderate radical scavenging activity relative to the strong antioxidant ascorbic acid (p-value <0.05), which demonstrated 85% radical scavenging activity at 100 μg/ml (Figure 1).
Furthermore, the ability of the A. palestina oil to reduce iron (III) to iron (II) was determined and compared to ascorbic acid, as shown in Figure 2. The results illustrate that the essential oil was able to reduce iron in a dose-dependent manner with a maximum reducing power of 65% at 100 μg/ml, whereas ascorbic acid resulted in 89% reduction efficiency of the sample (Figure 2), at the same concentration (p-value <0.05).
In addition, the hydroxyl (OH.) reactive oxygen species (ROS) is one of the most reactive and physiologically harmful free radicals. In this study, the concentration of the essential oil needed for scavenging 50% of the hydroxyl activity was found at 56 μg/ml, whereas that for the standard antioxidant, ascorbic acid, was at 45 μg/ml (Figure 3). Seemingly, the scavenging activity possessed by the essential oil is comparable with the strong antioxidant ascorbic acid (p-value <0.05).
Free radicals are the most common initiators of oxidative reactions that may result in numerous deleterious effects [24]. Natural antioxidants can scavenge and react with free radicals, and hence terminate the free radical reaction. Recently, herbal medicines containing free radical scavengers are drawing attention of the pharmaceutical research for their importance in preventing and treating several diseases and disorders [25]. Generally, essential oils were shown to protect against oxidative stress by contributing to the total antioxidant defense system of the human body [26]. Recently, a number of essential oils isolated from several medicinal and aromatic plants were shown to possess considerable antioxidant potential and, consequently, protect against some cardiovascular and degenerative diseases [26–28]. Our findings demonstrate that the essential oil of A. palestina has a substantial antioxidant potential that may be attributed to its chemical composition.
Furthermore, the oil from A. palestina was assayed for its in vitro antibacterial activity, using the microdilution method, towards six different bacterial species, representing both Gram positive and Gram negative bacterial (Table 1). Literature review revealed that there are no previous reports about the antibacterial activity of A. palestina. Our results indicated that the essential oil exhibited a broad-spectrum antibacterial activity. Interestingly, the results demonstrated that Gram-positive bacteria were more susceptible to the oil treatment than Gram-negative bacteria. Particularly, Staphylococcus epidermidis (MIC 6 ± 1 μg/ml) was the most susceptible Gram positive bacteria while Escherichia coli (MIC 53 ± 4 μg/ml) was the most affected Gram negative bacteria (Table 1).
Notably, the essential oil of A. palestina exhibited moderate antifungal activity against Candida albicans; with MIC value of 48 ± 5 μg/ml, Candida glabrata; with MIC value of 86 ± 4 μg/ml, and Candida krusei; with MIC value of 95 ± 3 μg/ml (Table 1). The increased incidence of systemic candidiasis infections, caused by pathogenic yeasts in critically ill patients [29], as well as the emergence of resistant Candida species to several antifungal drugs, necessitates a comprehensive search for newer drugs to treat candidiasis. Chemical diversity of medicinal plants may provide unlimited prospects for new therapeutic reagents discovery and development. Our findings indicate that the essential oil of A. palestina may have a potential for the development of new antifungal, as well as antibacterial agents, and demonstrate the importance of such medicinal plant in pharmaceutical production and industry.
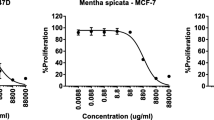
On the other hand, the plant hydrodistilled oil was further evaluated for its in vitro cytotoxic properties on eight human cancer cell lines, including human breast adenocarcinoma MCF-7 cell line, the human ductal breast epithelial tumor T47D cell line, the EBV-negative Burkitt’s lymphoma BJAB cell line, the human colon adenocarcinoma Caco-2 cell line, the human epithelial carcinoma HeLa cell line, the human prostate adenocarcinoma PC-3 cell line, the human clear cell renal cell carcinoma Caki cell line, and the human kidney carcinoma A498 cell line. It is known that different cell lines might demonstrate variable sensitivities to different plant extracts and, therefore, the use of more than one cell line appears compulsory for the comprehensive anti-cancer activity screening. Cytotoxic effects of the oil on the growth of the studied cell lines are presented in Figure 4, which shows the percentage of growth inhibition versus the concentration of the oil. The concentration of the oil at which growth was inhibited by 50% (LD50) was calculated based on the dose-dependent curves (Table 2). According to the demonstrated results, the oil exhibited potent in vitro cytotoxic properties on HeLa (LD50 32 μg/ml), Caco-2 (LD50 61 μg/ml), and BJAB cell lines (LD50 57 μg/ml). The variation in cytotoxic effects observed in different cell types is likely due to the versatility of the oil composition and the different ways the oil components may interact with different cell types [30]. Interestingly, the presence of 1,8-cineole, as one of the main oil constituents, supports its cytotoxic activity as suggested previously [31, 32].
Furthermore, literature reports on the composition-activity correlations were found in support with the findings of the present study. For example, in literature reviews concerning essential oils as active antimicrobials [33–35], it was reported that most of the antimicrobial activity of the essential oils is attributed to the oxygenated monoterpenes [35]. In particular, it was evident that monocyclic monoterpenes, such as 1,8-cineole and terpinen-4-ol, exhibit high antimicrobial activity against a wide range of Gram-positive and Gram-negative bacteria [33–35]. Additionally, sesquiterpenes caryophyllene, germacrene D, caryophyllene oxide, and spathulenol were also reported among the most potent antimicrobial agents frequently occurring in essential oils [33–35]. Nonetheless, since essential oils consist of multiple components, their antimicrobial activities cannot be assigned to one particular component. In most cases their activity is a result of additive, synergistic or antagonistic effects of the individual constituents. Therefore, the principle oil component is generally assumed to possess the strongest activity that can be influenced by the presence of other oil components.
Similarly, cytotoxic activities of essential oils from various aromatic plants were attributed to specific components of the oils [36, 37]. Some of the main components of A. palestina oil have previously been tested for cytotoxic properties. For example, β-caryophyllene was reported to be specifically cytotoxic to MCF-7, MDA-MB-468 and UACC-257 cancer cell lines [38].
On the other hand, antioxidant activity is one of the most intensively studied subjects in essential oil research [39, 40]. The oxygenated constituents, specifically phenolic monoterpenic alcohols (e.g. thymol and carvacrol), as well as phenylpropanoids (e.g. eugenol), are the most powerful antioxidant volatiles [41]. Nevertheless, some non-phenolic terpenoids were also reported to be of significant antioxidant capacities [41]. Additionally, Many other components of essential oils were proposed to contribute to the antioxidant activity of essential oils [42]; including α-terpinene, β-terpinene, β-terpinolene, 1,8-cineole, menthone, isomenthone, and citronellal [42]. Accordingly, the high percentage of oxygenated terpenoids and the particular presence of some alcohols, as 1,8-cineole and terpinen-4-ol, in the oil of A. palestina would rationalize its relatively high antioxidant activity.
Conclusions
In conclusion, our results revealed that the essential oil of Anthemis palestina exhibits considerable antioxidant activity and can be used as alternative medicine to prevent or treat oxidative stress. Furthermore, the examined oil demonstrates moderate antibacterial and antifungal activities elucidated by the growth inhibitory response against clinically problematic microorganisms. The discrepancy observed in the cytotoxicity activities of the oil against different cancer cell lines suggests a unique antiproliferative mechanism through which the oil exhibits its anticancer effects. Further research into the molecular mechanisms, as well as isolation and characterization of the chemical constituents responsible for the activity would be necessary to identify the active components with potential for clinical use.
References
Fernandes R: Genus Anthemis L. Flora Europaea. Volume 4. Edited by: Europaea F, Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb A. 1976, Cambridge, London: Cambridge University Press, 145-159.
Bremer K, Humphries CJ: Generic Monograph of the Asteraceae-Anthemideae. B Nat Hist Mus. 1993, 23: 71-177.
Bremer K: Asteraceae, Cladistics and Classification. 1994, Portland, Oregon: Timber Press
Javidnia K, Miri R, Kamalinejad M, Sarkarzadeh H, Jamalian A: Chemical composition of the essential oils of Anthemis altissima L. grown in Iran. Flavour Frag J. 2004, 19: 213-216.
Saroglou V, Dorizas N, Kypriotakis Z, Skaltsa HD: Analysis of the essential oil composition of eight Anthemi s species from Greece. J Chromatogr A. 2006, 1104: 313-322.
Rezaee MB, Jaimand K, Assareh MH: Chemical constituents of the leaf and flower oils from Anthemis altissima L. var. altissima from Iran. J Ess Oil Res. 2006, 18: 152-153.
Kivcak B, Mert T, Saglam H, Ozturk T, Kurkcuoglu M, Baser KHC: Chemical composition and antimicrobial activity of the essential oil of Anthemis wiedemanniana from Turkey. Chem Nat Comp. 2007, 43: 47-51.
Vaverkova S, Haban M, Eerna K: Qualitative properties of Anthemis tinctoria and Anthemis nobilis (Chamaemelum nobile) under different environmental conditions. J Cent Eur Agr. 2001, 2: 61-156.
Honda G, Yesilada E, Tabata M, Sezik E, Fujita T, Takeda Y, Tanaka T: Traditional medicine in Turkey. J Ethnopharmacol. 1996, 53: 75-87.
Konstantinopoulou M, Karioti A, Skaltsas S, Skaltsa H: Sesquiterpene lactones from Anthemis altissima and their anti-Helicobacter pylori activity. J Nat Prod. 2003, 66: 699-702.
Teixeira Da Silva JA: Mining the essential oils of the Anthemideae. African J Biotechnol. 2004, 3: 706-720.
Melegari M, Albasini A, Pecorari P, Vampa G, Rinaldi M, Rossi T, Bianchi A: Chemical characteristics and pharmacological properties of the essential oils of Anthemis nobilis. Fitoterapia. 1988, 59: 449-455.
Schrader A, Eckey H, Rohr M: Examination of the activity of irradiation-lowering substances on the human skin exemplified with various chamomile extracts. SOFW Journal. 1997, 123: 3-11.
Rudzki E, Jalblonska S: Kamillosan is a safe product of camomile for topical application: Results of patch testing consecutive patients with contact dermatitis. J Dermatol Treat. 2000, 11: 161-163.
Povilaityte V, Venskutonis PR, Jukneviciene G: Aroma and antioxidant properties of Roman chamomile (Anthemisnobilis L.). Food Flavors and Chemistry: Advances of the New Millennium. Edited by: Arthur MS. 2001, London, UK: Royal Society of Chemistry
Teixeira DMC, Glyn MF, Adilson S, Vera LGR, Demarmelina C: Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol. 2005, 97: 305-311.
Al-Eisawi DM: Field Guide to Wild Flowers of Jordan and Neighboring Countries. 1998, Foundation Al-Rai, Amman, Jordan: Jordan Press
Tawaha KA, Alali FQ, Hudaib MM: Chemical Composition and General Cytotoxicity Evaluation of Essential Oil from the Flowers of Anthemis palestina Reut. Ex Boiss. Growing in Jordan. J Ess Oil Bear Pl. In press
Sreejayan N, Rao MN: Free radical scavenging activity of curcuminoids. Drug Res. 1996, 46: 169-171.
Klein SM, Cohen G, Cederbaum AI: Production of formaldehyde during metabolism of Dimethyl sulphoxide by Hydroxyl Radical Generating System. Biochemistry. 1981, 20: 6006-6012.
Yen G, Chen H: Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995, 43: 27-32.
Clinical Laboratory Standards Institute: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Edited by: Wayne PA. 2006, Approved Standard M7-A7, 7
Tubaro A, Florio C, Luxich E, Vertua R, Della Loggia R, Yasumoto T: Suitability of the MTT based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon. 1996, 34: 965-974.
Antolovich M, Prenzler P, Patsalides E, McDonald S, Robards K: Methods for testing antioxidant activity. Analyst. 2002, 127: 183-198.
Dharmendra D, Prashant K, Jain SK: In-vitro Anti-oxidant activity of the ethyl acetate extract of gum guggul (Commiphora Mukul). Biological Forum-An Int J. 2009, 1: 32-35.
Erkan N, Ayranci G, Ayranci E: Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella Sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110: 76-82.
Kelen M, Tepe B: Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresource Technol. 2008, 99: 4096-40104.
Maria Graça M: Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules. 2010, 15: 9252-9287.
Meon M, Marchetti O, Calandra T: Bench-to bedside review: Candida infections in the intensive care unit. Crit Care. 2008, 12: 204-208.
Kamuhabwa A, Nshimo C, de Witte P: Cytotoxicity of some medicinal plant extracts used in Tanzanian traditional medicine. J Ethnopharmacol. 2000, 70: 143-149.
Moteki H, Hibasami H, Yamada Y, Katsuzaki H, Imai K, Komiya T: Specific induction ofapoptosis by 1,8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncology Rep. 2002, 9: 757-760.
Conforti F, Menichini F, Formisano C, Rigano D, Senatore F, Bruno M, Rosselli S, Celik S: Anthemis wiedemanniana essential oil prevents LPS-induced production of NO in RAW 264.7 macrophages and exerts antiproliferative and antibacterial activities in vitro. Nat Prod Res. 2012, 26: 1594-1601.
Lang G, Buchbauer G: A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review Flav Frag J. 2012, 27: 13-39.
Baratta MT, Dorman HJ, Deans SG, Figueiredo AC, Barroso JG, Ruperto G: Antimicrobial and antioxidant properties of some commercial essential oils. Flav Frag J. 1998, 13: 235-244.
Knoblach K, Pauli A, Iberi B, Wegand H, Weis N: Antibacterial and antifungal properties of essential oil components. J Ess Oil Res. 1989, 1: 119-128.
Bhardwaj P, Alok U, Khanna A: In vitro cytotoxicity of essential oils: A review. Int J Res Pharm Chem. 2013, 3: 675-681.
Ben Chobba I, Bekir A, Ben Mansour R, Drira N, Gharsallah N, Kadri A: In vitro evaluation of antimicrobial and cytotoxic activities of Rosmarinus officinalis L. (Lamiaceae) essential oil cultivated from south-west Tunisia. Appl Pharmaceut Sci. 2012, 2: 34-39.
Setzer WN, Schmidt JM, Noletto JA, Vogler B: Leaf oil compositions and bioactivities of abaco bush medicines. Pharmacologyonline. 2006, 3: 794-802.
Amorati R, Foti MC, Valgimigli L: Antioxidant Activity of Essential Oils. J Agric Food Chem. 2013, 61: 10835-10847.
Lee K-G, Shibamoto T: Determination of Antioxidant Potential of Volatile Extracts Isolated from Various Herbs and Spices. J Agric Food Chem. 2002, 50: 4947-4952.
Ruberto G, Baratta MT: Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2006, 69: 167-174.
Shaaban H, El-Ghorab A, Shibamoto T: Bioactivity of essential oils and their volatile aroma components: Review. J Ess Oil Res. 2012, 24: 203-212.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6882/14/297/prepub
Acknowledgements
The authors would like to thank The Deanship of Academic Research at The University of Jordan for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SB designed and performed the experiments, interpreted the results, drafted the manuscript and revised it. KT collected the plant, isolated the essential oil, and participated in the design of the study. MH discussed and evaluated the results, performed the statistical analysis and participated in writing the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bardaweel, S.K., Tawaha, K.A. & Hudaib, M.M. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement Altern Med 14, 297 (2014). https://doi.org/10.1186/1472-6882-14-297
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6882-14-297