Abstract
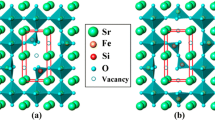
The kinetics of oxygen exchange in the nonstoichiometric strontium ferrite SrFeO3–δ with the structure of cubic perovskite was studied by the oxygen partial pressure relaxation technique in the isostoichiometric regime at temperatures of 500–900°C in a range of δ from 0.24 to 0.44. An increase in the oxygen nonstoichiometry δ was accompanied by a decrease in the rate of sample relaxation and by an increase in the apparent activation energy of oxygen exchange.
Similar content being viewed by others
References
Diethelm, S., Closset, A., Van herle, J., McEvoy, A.J., and Nisancioglu, K., Solid State Ionics, 2000, vol. 135, p. 613.
Bredesen, R., Mertins, F., and Norby, T., Catal. Today, 2000, vol. 56, p. 315.
Lane, J.A. and Kilner, J.A., Solid State Ionics, 2000, vol. 136, p. 997.
Kim, S., Yang, Y.L., Jacobson, A.J., and Abeles, B., Solid State Ionics, 1998, vol. 106, p. 189.
Sunde, S., Nisancioglu, K., and Gur, T.M., J. Electrochem. Soc., 1996, vol. 143, p. 3497.
Yoo, J., Verma, A., Wang, S., and Jacobson, A.J., J. Electrochem. Soc., 2005, vol. 152, p. A497.
Crank, J., Mathematics of Diffusion, Oxford: Oxford Univ. Press, 1995, 2nd ed., p. 60.
Starkov, I.A., Bychkov, S.F., Chizhik, S.A., and Nemudry, A.P., Chem. Mater., 2014, vol. 26, no. 6, p. 2113.
Savinskaya, O.A., Nemudry, A.P., Nadeev, A.N., and Tsybulya, S.V., Solid State Ionics, 2008, vol. 179, p. 1076.
Markov, A.A., Leonidov, I.A., Patrakeev, M.V., Kozhevnikov, V.L., Savinskaya, O.A., Ancharova, U.V., and Nemudry, A.P., Solid State Ionics, 2008, vol. 179, p. 1050.
Conder, K., Pomjakushina, E., Soldatov, A., and Mitberg, E., Mater. Res. Bull., 2005, vol. 40, p. 257.
Tofield, B.C., Greaves, C., and Fender, B.E.F., Mater. Res. Bull., 1975, vol. 10, no. 7, p. 737.
Haavik, C., Atake, T., and Stolen, S., Phys. Chem. Chem. Phys., 2002, vol. 4, p. 1082.
Hodges, J.P., Short, S., Jorgensen, J.D., Xiong, X., Dabrowski, B., Mini, S.M., and Kimball, C.W., J. Solid State Chem., 2000, vol. 151, p. 190.
Takeda, Y., Kanno, K., Takeda, T., and Yamamoto, O., J. Solid State Chem., 1986, vol. 63, p. 237.
Bakken, E., Stolen, S., Norby, T., Glenne, R., and Budd, M., Solid State Ionics, 2004, vol. 167, p. 367.
Mizusaki, J., Okaysu, M., Yamauchi, S., and Fueki, F., J. Solid State Chem., 1992, vol. 99, p. 166.
Starkov, I., Bychkov, S., Matvienko, A., and Nemudry, A., Phys. Chem. Chem. Phys., 2014, vol. 16, p. 5527.
Bouwmeester, H.J.M., Kruidhof, H., and Burggraaf, A.J., Solid State Ionics, 1994, vol. 72, p. 185.
Jacobson, A.J., J. Electrochem. Soc., 2005, vol. 152, p. A497.
Underwood, E.E., Quantitative Stereology., Reading, Mass.: Addison–Wesley, 1970, p. 23.
Virkar, A.V. and Wright, J., Symp. on Mixed Ionic Electronic Conductors, Seattle, Wash., 2012.
Ganeshananthan, R. and Virkar, A.V., High Temperature Materials Chemistry Symp. in Honor of Prof. C. B. Alcock, Orlando, Fla.,2003.
Ganeshananthan, R. and Virkar, A.V., J. Electrochem. Soc., 2005, vol. 152, no. 8, p. 1620.
Patrakeev, M.V., Leonidov, I.A., Kozhevnikov, V.L., and Kharton, V.V., Solid State Sci., 2004, vol. 6, p. 907.
Huang, K. and Goodenough, J.B., J. Electrochem. Soc., 2001, vol. 148, p. E203.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.F. Bychkov, M.P. Popov, A.P. Nemudry, 2016, published in Kinetika i Kataliz, 2016, Vol. 57, No. 5, pp. 705–710.
Rights and permissions
About this article
Cite this article
Bychkov, S.F., Popov, M.P. & Nemudry, A.P. Study of the oxygen exchange kinetics in the nonstoichiometric oxide SrFeO3–δ under isostoichiometric conditions using the oxygen partial pressure relaxation technique. Kinet Catal 57, 697–703 (2016). https://doi.org/10.1134/S0023158416050050
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158416050050