Abstract
Anterior uveitis (AU), inflammation of the iris, choroid or ciliary body, can cause significant eye morbidity, including visual loss. In the pediatric age group, the most common underlying diagnosis for AU is juvenile idiopathic associated uveitis and idiopathic AU, which are the focus of this paper. AU is often resistant to medications such as topical corticosteroids and methotrexate. In the past 15 years, biologic agents (biologics) have transformed treatment. In this review, we discuss those in widespread use and those with more theoretical applications for anterior uveitis. Tumor necrosis factor alpha inhibitors (anti-TNFα) have been available the longest and are used widely to treat pediatric uveitis. The effects of anti-TNFα in children are described mostly in small retrospective case series. Together, the literature suggests that the majority of children treated with anti-TNFα achieve decreased uveitis activity and reduced corticosteroid burden. However, many will have disease flares even on treatment. Only a few small studies directly compare outcomes between alternate anti-TNFα (infliximab and adalimumab). The use of different uveitis grading systems, inclusion criteria, and outcome measures makes cross-study comparisons difficult. Whether the achievement and maintenance of inactive disease occurs more frequently with certain anti-TNFα remains controversial. Newer biologics that modulate the immune system differently (e.g., interfere with Th17 activation through IL-17a and IL-6 blockade, limit T lymphocyte costimulation, and deplete B lymphocytes), have shown promise for uveitis. Studies of these agents are small and include mostly adults. Additional biologics are also being explored to treat uveitis. With their advent, we are hopeful that outcomes will ultimately be improved for children with AU. With many biologics available, much work remains to identify the optimal inflammatory pathway to target in AU.
Similar content being viewed by others
Tumor necrosis factor-α (TNFα) inhibitors have been used successfully to treat steroid- and methotrexate-resistant uveitis. |
The comparative effectiveness of different TNFα inhibitors has not been well described. |
Newer biologics are expected to play a growing role in the treatment of pediatric uveitis. |
1 Introduction
Anterior uveitis (AU), inflammation of the iris, choroid, and/or ciliary body, can be idiopathic or secondary to an underlying autoimmune condition. It carries significant morbidity, most importantly the risk of decreased visual acuity or blindness. While corticosteroids (CS) and methotrexate (MTX) have historically been primary treatment options, in the past 15 years biologic agents (biologics) have transformed our approach to treatment. In this review, we discuss those biologics currently in widespread use and those with more theoretical applications for juvenile idiopathic arthritis (JIA)-associated and idiopathic AU.
Uveitis may be restricted anatomically to the anterior chamber (AU), intermediate chamber (intermediate uveitis, IU) or posterior chamber (including the retina) or can involve multiple regions (panuveitis) [1]. In 2000, Cunningham [2–4] described posterior uveitis as the most prevalent type in children (40–50 %), but it is now recognized that AU is the most prevalent type (56.9–58.4 %). Some of this discrepancy may depend on the population (posterior more prevalent in tertiary care facilities) and the age group studied [5]. In a British study, chronic AU was the most common in children <7 years old, posterior uveitis in 8–15 year olds, and acute AU in 16–19 year olds [5].
In addition to being classified by anatomic location, there are other clinically important descriptors of uveitis. As described by the Standardization of Uveitis Nomenclature (SUN) Working Group (see below), uveitis is classified as unilateral or bilateral; sudden or insidious in onset; limited (≤3 months) or persistent (>3 months) in duration; and acute, recurrent or chronic [1]. When disease relapses within 3 months of discontinuing treatment, it is classified as chronic [1]. Unique patterns are associated with underlying systemic diseases. For example, uveitis associated with JIA (JIA-U) is most often an insidious (because it is asymptomatic), chronic relapsing AU that affects both eyes over time [6], whereas other types of non-JIA-U may more frequently be acute and symptomatic (eye pain, redness, and/or change in vision). Idiopathic, or undifferentiated, uveitis may also be chronic and bilateral, although it more often primarily affects the intermediate chamber. Notably, uveitis localized to a particular segment may also ‘spill over’ to involve other areas.
There has historically been great variation in the assessment of AU activity. What defined inactive disease varied in the literature, as did assessment of degree of inflammation. Neither was there uniformity in the assessment of change in uveitis activity [7–10]. This resulted in difficulty comparing outcomes between studies. In an attempt to address this and facilitate more interpretable data for research, a group of experts formed the SUN Working Group. In 2005, they published uveitis consensus guidelines [1]. These included grading scales for anterior chamber (AC) cell (based on the number of cells in 1 mm slit-lamp beam) and AC flare (Table 1). The guidelines include terminology descriptors for inactive disease, worsening disease, improving disease, and remission [1] (Table 1). Subsequently, clinicians and researchers have worked to integrate these definitions. Over time, universal adoption of a single grading language will help comparative effectiveness research in uveitis. The SUN Working Group deferred to previously published guidelines to describe intermediate (vitritis) and posterior uveitis [11]. As AU can be associated with posterior involvement that is not detected by the slit lamp exam, all patients with chronic uveitis should be screened for macular edema and epiretinal membranes through the use of optical coherence tomography [1, 12, 13].
Comprehensive incidence studies of childhood uveitis are lacking, and reported incidences vary. Most studies have been performed in geographically restricted or non-ethnically diverse populations. These have described the incidence of non-infectious uveitis to range from 4 to 6 per 100,000 in children younger than 15 or 16 years old, but to rise to 3–11/100,000 in adolescents [5, 14, 15]. Possibly the largest and most ethnically diverse population-based study utilized the database of a large Northern California Health Maintenance Program (Kaiser Permanente) [16]. This estimated the incidence of any type of eye inflammation (not restricted to non-infectious uveitis) in children younger than 15 years as 7/100,000, and 27/100,000 in adolescents [16].
Uveitis can be infectious, related to an underlying disease, or be idiopathic (a diagnosis of exclusion). Associated systemic illnesses include JIA, tubulointerstitial nephritis and uveitis (TINU), sarcoidosis, Behçet disease, Vogt–Koyanagi–Harada syndrome, Cogan syndrome, juvenile xanthogranuloma, post-transplant lymphoproliferative disease, and leukemia [2, 17]. While idiopathic uveitis may be the most common overall (21.5–78 %) [4, 5], JIA is the most common identifiable cause of AU, especially in younger children, comprising 19–67 % of uveitis, depending on whether the study population is based in primary care or referral centers or is population based [2–5]. Amongst those with JIA, 12–20 % of children develop uveitis [6, 18–20], most frequently chronic AU [6]. While some studies of uveitis therapies are restricted to children with JIA-U, others include children with all non-infectious uveitis types. Amongst these, idiopathic uveitis is the most frequent. We will focus our discussion on these most common causes of pediatric non-infectious AU, JIA-associated and idiopathic uveitis.
2 Risk Factors for Uveitis in Juvenile Idiopathic Arthritis (JIA)
The rate of uveitis is highest amongst children with extended oligoarticular JIA (25 %) [18]. Various risk factors for uveitis in JIA have been reported: JIA subtype, female sex, younger age at onset of arthritis, and anti-nuclear antibody (ANA)-positivity [20–23]. These factors are reflected in the American Academy of Pediatrics screening guidelines in which ANA status, JIA subtype, and age of onset drive the recommended frequency of uveitis screening in children with JIA [24]. More recent work suggests that interactions between features may be most important, in that development of uveitis in girls who were younger than 3 years old at JIA onset (but not in males) and ANA positivity (rather than subtype) were risk factors for uveitis [19], while newer work supports the notion that only younger age of onset and ANA status are risk factors, but not subtype or sex [18, 25].
3 Potential Complications
AU can result in numerous eye complications. While posterior synechiae [3, 18, 26] and band keratopathy [3, 18, 26] are the most common, other, possibly sight threatening, complications include cataracts [3–5, 18, 26], increased intra-ocular pressure [3–5, 18, 26], cystoid macular edema [2, 3, 26], neovascularization of the retina, and amblyopia [2, 27]. Complications can be caused by inflammation and/or by CS used to treat disease (e.g., glaucoma, cataracts). Relative to adults with uveitis, children may be at increased risk for developing CS-induced cataracts and increased intra-ocular pressure as well as at unique risk for systemic CS-induced growth retardation [2]. Loss of visual acuity is a result of these complications and is the most dire outcome of uveitis.
Certain factors have been positively associated with the development of complications. Amongst all uveitis, those with a diagnosis of JIA have the highest risk [4]. This may be attributable to the asymptomatic nature of JIA-U and therefore a delay in coming to medical attention, as well as the chronic nature of this disease [5]. In studies of children with JIA-U, 67 % had complications at the outset [28], and 37–56 % of patients had complications at some time during follow up [6, 18].
Amongst those with JIA-U, the following factors have been associated with increased risk of complications and/or disease severity: complications at the outset [18]; male sex [29]; severe disease [30]; AC cell ≥1+ at presentation [31]; ≥1+ AC flare [28, 32]; positive ANA [28]; shorter duration between the diagnosis of arthritis and uveitis [6, 29, 33]; and uveitis before arthritis [18]. In particular, posterior synechiae, active uveitis, and the use of topical CS (tCS) are risks factors for the development of cataracts [34]. The incidence of cataract, during 3 years of follow up, was decreased by 87 % with use of ≤3 drops per day (gtt/day) of tCS relative to >3 gtt/day [35]. Because the point estimate of cataract was 0/EY (eye-year) using ≤2 gtt/day [35], many uveitis experts use this as a cut-off for a tolerable level of chronic tCS use.
Historically, up to a third of children with uveitis were reported to be visually impaired [2]. In 1967, 36 % of children with JIA in a Toronto clinic had vision worse than 20/200 [36]. Final visual acuity was worse than 20/200 in 26 % of eyes in patients with juvenile rheumatoid arthritis (JRA) from the Massachusetts Eye and Ear Infirmary (1982–1994) [4]. In a Baltimore-based cohort of JIA-U, 36 % of children had visual impairment (20/50 or worse), and 24 % had vision loss (20/200 or worse) at presentation (1984–2005) [28]. However, estimates, even for less severe vision impairment, have been lower in other recent cohorts—between 3.4 % of children with JIA to 17 % of all children with uveitis have had visual acuity worse than 20/40 [5, 6]. Improved visual outcomes most likely reflect earlier identification of disease, through implementation of JIA ophthalmological screening, as well as improved therapeutics.
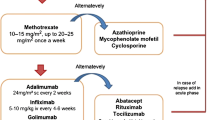
4 Non-Biologic Uveitis Treatment
Topical corticosteroids are the first line of treatment for AU [37, 38]; intermediate and posterior uveitis require peri-ocular or intra-ocular CS injections/depots or systemic CS. Yet tCS are frequently inadequate to control disease chronically, either from lack of efficacy, inability to taper, or development of adverse effects. When uveitis persists despite tCS (CS resistant), when children develop CS side effects (CS intolerant), or when uveitis worsens with attempts at tCS taper (CS dependent), immunomodulators are added. Specific guidelines for when to add these immunomodulators do not currently exist. Most aim to avoid systemic CS unless there is severe inflammation causing vision loss, lack of adherence to topical regimens, and/or if the posterior segment is involved, as tCS do not reach the posterior segment. Conventional immunomodulators include antimetabolites such as MTX [39–44], mycophenolate mofetil (MMF) [45], azathioprine (AZA) [46], leflunomide [47, 48], and, less frequently, cyclosporine A (CsA) [49, 50]. Unless there are specific contraindications (decreased renal function or liver abnormalities), MTX is the most common and oldest immunomodulatory agent used for AU [37, 38] and is moderately effective [39–44]. While the true effectiveness of MTX in comparison with untreated controls is not reported, studies evaluate success in individuals whose uveitis was unable to be controlled, who were CS dependent, or who developed CS toxicity, and have shown benefit from MTX. In adults, 56–76 % of those with chronic uveitis achieved control, where control was defined as <1+ cellular reaction for ≥6 months (AU) [43] or no inflammation sustained over two visits at least 28 days apart [40], although children were a third as likely as adults to achieve control [40]. It is recognized that MTX has a delayed onset of action and may take over 6 months to achieve full efficacy [40]. Reports focusing on the pediatric age group have been hampered by small numbers, different dosing regimens of MTX, and their retrospective nature. Foeldvari and Wierk [39] described no uveitis activity off all tCS a mean of 4.25 months after initiation of MTX in 21/25 children (84 %) with JIA-AU. Malik [42] described ten children treated with MTX for AU, with improvement in 6/10 (60 %) (no AC cells in 4/10) and decreased tCS use. Heiligenhaus et al. [41] reported that 21/31 children (68 %) with JIA-U had no inflammation after initiation of MTX without need for initiation of tCS or addition of another immunomodulating agent. Kalinina Ayuso et al. [51] reported 18/22 children (82 %) with JIA-U had improvement (SUN criteria) in uveitis 1 year after initiation of MTX, with 15 (68 %) achieving no AC cells by a median of 6 months. In 13 of these children, MTX was stopped due to inactive disease and 9/13 developed a relapse of inflammation a mean of 7.5 months after discontinuation of MTX. Relapses were less common in children older than 8 years of age, those who had been treated for longer than 3 years, or those who had been in remission for at least 2 years.
A recent meta-analysis of nine retrospective case series encompassing 135 children with chronic AU (majority JIA-U) resistant to steroid therapy calculated that 73 % (95 % confidence interval [CI] 66–81) of children responded to MTX, where response was assessed utilizing SUN criteria, although data was not available to calculate the effect estimate for its steroid-sparing benefit [52]. Recently published German guidelines recommended either MTX or AZA as first-line immunomodulation [37, 38]. However, when uveitis is not controlled on tCS and one immunomodulatory therapy (IMT), children develop cataracts or glaucoma in response to CS, children are unable to tolerate IMT due to side effects, or there is severe sight-threatening inflammation or eye complications early on, it has become standard of care to initiate treatment with a biologic medication, most often a tumor necrosis factor alpha inhibitor (anti-TNFα). This review will describe the clinical experience to date with biologics (Table 2). Searches were performed with the search terms ‘uveitis’ and ‘pediatric or juvenile’ crossed with ‘tumor-necrosis factor inhibitor’, ‘anti-TNF’, ‘etanercept’, ‘infliximab’, ‘adalimumab’, ‘abatacept’, ‘rituximab’, ‘tocilizumab’, ‘IL-17’, ‘Th-17’, ‘secucinumab’, ‘IL-12’, ‘IL-23’, ‘IL-1’, ‘anakinra’, ‘canakinumab’. Papers were selected that discussed treatment of JIA-U and idiopathic uveitis.
As the reader critically interprets the literature on uveitis treatment, it is critical to keep in mind that studies use different uveitis grading systems [1, 7–10], inclusion criteria, and outcome measures. Some studies include any child or adolescent with uveitis while others are restricted to patients with JIA-AU. In some studies, all patients had active uveitis at the onset of treatment, whereas in others uveitis was not active because of the use of high doses of concomitant therapies (CS and IMT). Accordingly, the outcome measures in some studies only include uveitis activity, sometimes measured indirectly, while in others they take into account the ability to taper other therapies. Alternatively, visual acuity and complications are included along with uveitis activity as outcome measures in some but not other studies.
5 Tumor Necrosis Factor-α Blockade
Since Federal Drug Administration (FDA) approval in the US in the late 1990s, physicians have used anti-TNFα to treat uveitis that is resistant to steroids and other conventional IMT. TNFα was initially identified as an inducer of apoptosis, but has since been recognized to have pleiotropic roles, including in proliferation, inflammation, angiogenesis, adhesion molecule expression, and even inhibition of regulatory T cells (Treg) [53, 54]. Produced by macrophages, natural killer (NK) cells, and B and T lymphocytes, it exerts its effects through interaction with TNF receptors. TNFα may be elevated in the serum and synovial fluid of patients with rheumatoid arthritis (RA). Anti-TNFα therapy is recommended for both RA and JIA treatment by the American College of Rheumatology [55, 56]. While the use of anti-TNFα to treat uveitis may have been a de facto outgrowth of its use in arthritis, there is a biologic rationale to TNF blockade in uveitis. For example, when TNF-receptor-IgG was administered in a murine model of experimental autoimmune uveitis, it limited damage to the experimentally targeted retinal rods [57]. Moreover, in humans with uveitis, levels of TNF have been shown to be elevated intra-ocularly and in the peripheral serum [58].
This is a constantly expanding medication class. The three anti-TNFα that have been used in pediatric uveitis are etanercept, infliximab, and adalimumab. Etanercept is a soluble receptor, comprised of a dimeric fusion protein of the human p75 TNF receptor linked to the Fc end of human IgG1. Etanercept binds to, and sequesters, soluble TNFα. Conversely, the other two are monoclonal antibodies to TNFα which block both membrane-bound and soluble TNF. Infliximab is a chimeric anti-TNFα antibody (human Fc and murine variable region). Adalimumab is a fully humanized IgG1 antibody to soluble and transmembrane TNFα. Both etanercept and adalimumab are administered by subcutaneous injection while infliximab is administered intravenously.
5.1 Etanercept
The earliest reports of anti-TNFα for pediatric uveitis examined the usefulness of etanercept (Table 3). Reiff [59] described ten children with AU (16 eyes) who were treated with twice weekly etanercept, after having failed treatment with tCS and MTX (or CsA). Using the Hogan classification system (includes AC cell and flare, vitreous cell, degree of cataract), by 3 months, 63 % of eyes (36 % by 6 months) had decreased AC cell, although very few were inflammation free. The effect was less robust in a study of 16 individuals treated with etanercept and MTX. Inflammation only improved in one of the three children (one JRA-AU, one idiopathic AU, one JRA-panuveitis) [60]. Of concern, amongst individuals who had begun etanercept for their joint disease, five developed eye inflammation. Development of uveitis in children on etanercept was also shown by another group in which two of 48 children treated with etanercept for articular disease developed uveitis [61]. Finally, a small randomized controlled trial was performed in which, after 6 months, the achievement of improvement in AC inflammation (SUN inflammation <1+ with <3 drops tCS per day or decrease in immunosuppression by 50 %) was the same in seven children treated with etanercept as in the five treated with placebo (standard of care with tCS, oral CS, and MTX) [62]. Etanercept is no longer considered a suitable treatment for JIA-U.
5.2 Infliximab
Researchers were concurrently examining the utility of infliximab in uveitis (Table 4). In 2005, Richards et al. [63] reported that inflammation resolved (Nussenblatt system, similar to SUN) in six children with JIA-AU treated with infliximab (5–10 mg/kg week 0, 2, 4 and then every 6–8 weeks); children were also able to decrease other IMTs. That year, Rajaraman et al. [64] described six children in whom inflammation (uveitis, pars planitis and papillitis) resolved entirely to an AC and vitreous activity of 0.5 or less, according to Foster and Vitale criteria, after treatment (infliximab 5–10 mg/kg). In almost all, inflammation improved after the first dose of infliximab, and disease remained quiet after discontinuing both oral and topical CS (83 %).
Four larger series in 2006–2007 described success in about three-quarters of those treated with infliximab. Kahn et al. [65] described 17 patients with uveitis (anterior, intermediate or panuveitis) treated with higher dose infliximab (10–20 mg/kg). Response was considered to be elimination of inflammation (<5 cells in AC, no vitreous haze or cell according the scoring system of BenEzra) but did not take into account an assessment of CS use [7]. All of the patients responded by the seventh dose, 76 % responded within the first two. Because CS were weaned as uveitis improved, 88 % of patients were able to discontinue tCS by the end of follow-up (median 13 months, range 3–34 months) as did 5/5 patients on oral CS. Those with the slowest responses were treated initially with ≤10 mg/kg, whereas those treated with 15–20 mg/kg responded after the first dose. Sobrin et al. [66] also described a good response to infliximab in 27 adults and children with ocular inflammatory disease (AU, panuveitis, and scleritis) (infliximab 5 mg/kg every 2–8 weeks, after loading). Using an outcome measure of inflammation based on SUN, 78 % of patients achieved improved activity (to no inflammation) (eight of the eight individuals with AU initially achieved control, but three relapsed). Of the entire cohort, 26 % of patients were able to achieve improved inflammation and stop other IMT and 33 % were able achieve improved inflammation and decrease IMT; the authors do not comment on the use of tCS. In life-table analysis, a majority of eyes (81–84 %) achieved control by 9 months.
Ardoin et al. [67] reported that 64 % of 16 children with active uveitis (50 % AU), or on oral CS for uveitis, achieved zero inflammation within 1 year after initiation of infliximab (median maintenance dose of 8.2 mg/kg every 5.6 weeks). Although this response rate is lower than that in the two studies above, the outcome measures were more stringent; when success was defined as no inflammation or a two-step decrease in inflammation, 79 % achieved success at 1 year. This group specifically addresses tCS use, as 69 % of patients were able to completely stop them by 1 year. Tugal-Tutkun et al. [68] also described a similar success in a group of 17 Turkish children with AU, in whom 76 % achieved disease inactivity by 10 weeks (82 % achieved inactivity or a 2-step decrease in steroids). While infliximab was administered at 5 mg/kg week 0, 2, and 6 and then every 8 weeks, in order to maintain control the dosing interval was shortened to every 4–6 weeks in four patients.
Sharma et al. [69] was the first to describe the success of infliximab even in children with AU who had failed alternate anti-TNFα (5/6 children). At 3 months, 50 % had achieved no inflammation (SUN criteria) and 83 % had achieved either improved or inactive inflammation. While systemic steroid doses were decreased, all patients remained on systemic steroids (3 on 5 mg/day); the doses of tCS are not described. While three patients had JIA, none had oligoarticular JIA; rather, all three had the psoriatic subtype; the remainder had HLA B27 positivity without a JIA diagnosis, Sarcoid disease or idiopathic granulomatous uveitis.
While Simonini et al. [70] reported similarly positive outcomes in 15 Italian children with chronic uveitis (by AC cell and/or vitreous haze), the authors concluded that infliximab had limited durability in controlling uveitis. Eighty-seven percent of children (13/15) achieved complete remission within 1 year (10-week median), and all 13 were able to discontinue systemic steroids within 3 months of initiation of infliximab (tCS use was not described). Once remission was achieved, the interval between infliximab doses was increased. Each child who achieved complete remission relapsed after 1 year. This rate of relapse is higher than that reported by two other groups in which, after achievement of control, uveitis reactivated by 1 year in only 25 % [68] or 42 % [67]. The higher relapse rate in the Italian cohort may be attributable to the use of lower doses of infliximab at increasingly less frequent intervals, a risk factor for the development of human anti-chimeric antibodies (HACA) and subsequent drug tolerance [71–73]. This highlights one of the difficulties posed by the use of differing outcome measures in studies of uveitis therapeutics. For example, readers must distinguish whether a study analyzes the percentage of patients in quiescence at 1 year or who have achieved quiescence by 1 year. The former outcome does not convey whether patients have been in and out of quiescence multiple times, and the latter outcome does not evaluate whether patients achieved quiescence at 3 months and then relapsed before 1 year.
5.3 Adalimumab
After adalimumab was approved by the FDA for use in JIA, there was interest in using the drug to treat uveitis (Table 4). In the first prospective trial describing the use of adalimumab for pediatric AU, inflammation improved, mostly to quiescence, in 81 % of eyes (93 % of 14 patients) with a rapid median response of 6 weeks [74]. The response was maintained in 65 % of eyes for the duration of the follow-up period. Many children were able to decrease their IMT, and 79 % decreased, and 29 % discontinued, tCS. The study’s outcome measure was distinct from that in many others in that definition of inflammation included either AC cell or AC flare. Utilization of flare as an outcome measure has been controversial, mainly because it is difficult to assess clinically [32, 75], and most other studies have focused on AC cell alone. In another study of 18 patients with AU (17 JIA-U), 13 of whom had previously failed other anti-TNF, adalimumab was “effective” in 83 % of patients—who were also able to discontinue systemic steroids [76]. Again, the outcome measure was problematic as it connoted the percentage of patients who achieved a decrease in the number of uveitis relapses while on adalimumab relative to before treatment, rather than any description of eye inflammation. A similar level of success was also reported in a study of 14 Spanish children with active uveitis (most AU) in which 12 (86 %) had improvement, by SUN criteria, in uveitis within 4 weeks (median), although patients’ ability to wean systemic medications or tCS is not described [77].
Other studies show less success. When five children with uveitis (location not specified) were treated with adalimumab, only 50 % of eyes improved when the outcome measure was ≥1 grade improvement in the Foster and Vitale definition (inactivity: <0.5 AC cell and flare and vitreous cells, each on 0–4 scale with gradations of 0.5); neither complete resolution of inflammation nor weaning of CS were included as endpoints (80). In a study of 20 Finnish children, Tynjälä et al. reported an even less impressive response—only 20 % of 40 eyes with AU (35 % of patients) improved with adalimumab therapy (SUN criteria) and were able to stop systemic CS [79]. In another larger Finnish study of 54 children with AU, after 2 years of treatment only one third were under “good control”, 31 % by paper-specific criteria (<3 cell/hpf and no tCS) or 28 % with improved activity by SUN criteria [80]. Another third were under “moderate control” by paper-specific criteria (35 %, 3–9 cell/hpf and ≤3 tCS/day). Oral CS were only discontinued in 22 %, and most children remained on at least one additional form of IMT. To achieve control, almost one half of patients had increased from fortnightly to weekly adalimumab. Five patients stopped due to inefficacy or adverse effects. It is unlikely that these less promising results are because patients had already failed treatment with other TNF—this had also been the case in the Biester et al. cohort [76]. However, none of the studies evaluate whether the response rate to adalimumab varies by previous TNF exposure (confounding by indication).
The study by Biester et al. [76] suggested that adalimumab did not only help control uveitis but that it was beneficial in sustaining that response. Despite less than promising results regarding achievement of control, Tynjälä et al. [79] also reported that there was a statistically significant decrease in the mean number of disease flares per year while on adalimumab relative to before treatment, from 1.9 to 1.4 (p = 0.039). In contradistinction, in a study of 17 children with AU in which 31 % achieved no active inflammation by 3 months (and 50 % of eyes had improved inflammation by SUN criteria), almost a third of eyes had relapsed at 12 months, and there was a high requirement for systemic CS, periocular steroids, or the addition of conventional IMT (MMF) [81]. While many Spanish children relapsed less often while on adalimumab than they had before treatment, this was not the case in 40 % of children [77]. As a result, the authors suggest that adalimumab becomes less effective over time.
5.4 Is one anti-TNFα preferable over the other?
As the outcome measures utilized vary between studies, it is challenging to compare the effectiveness of different agents (Table 4). There is general consensus that etanercept is not effective for uveitis, and, as such, it will not be further considered in this section [82–84]. In the previously described Gallagher study, 13 children were treated with infliximab and five with adalimumab [78]. Inflammation improved in 20/26 (77 %) of eyes on infliximab (700–100 mg every 6–8 weeks) vs 5/10 (50 %) on adalimumab. Similarly, infliximab treatment resulted in improved visual acuity in more patients (62 vs 40 %). Despite infliximab’s apparent benefit, the speed to response was more rapid with adalimumab, median time to decreased inflammation 3.9 vs 10 weeks. Importantly this study was not powered to compare drug effectiveness, and patients were not randomized; the authors do not claim superiority of either drug.
Conversely, authors of a study using the National Italian Registry compared outcomes of children with JIA-AU treated with both medications (n = 48 infliximab, n = 43 adalimumab), and concluded that adalimumab worked better in the “medium-term period” (1 year) [85]. Using descriptive statistics, the authors reported that a greater percentage of children achieved remission on adalimumab (67.4 vs 42.8 %), as defined by no activity for 6 months on systemic medications (IMT and/or systemic CS) and on fewer than one drop/day tCS. Notably, only 16/35 patients were able to discontinue systemic, and 38/91 topical, CS. In a smaller prospective study also conducted in an Italian cohort, the authors examined whether either drug had a greater ability to sustain quiescence once it was achieved (n = 17 infliximab, and n = 16 adalimumab) [86]. They suggest, using survival analysis, that adalimumab leads to a more durable control. At 40 months, a greater percentage of children treated with adalimumab remained in quiescence (60 vs 18.8 %), while such differences were not present at the 12-month time point. Notably, the median follow up was significantly shorter in the adalimumab group (22 vs 31 months) with a maximum follow up of 36 months. There were no significant differences in the time to attain quiescence or to quiescence-with-discontinuation-of-steroids (median 3 months for both), although the authors do not clarify whether they are including discontinuation of tCS in this measure. In a small study with 2 years of follow up, Doycheva et al. [82] also reported an improved control of uveitis (0 AC cells while on ≤2 drops/day tCS) in children with ANA-associated AU under adalimumab (18/23 patients, 78 %) relative to infliximab (2/5, 40 %). In all three studies, patients were treated with low-dose infliximab (3–5 mg/kg in weeks 0, 2, 6, and then every 6–8 weeks), whereas higher doses of infliximab, up to 20 mg/kg every 4 weeks, were used in previous studies. Lower doses and increased intervals may limit the benefits from infliximab [87] and increase the likelihood of HACA development [65, 71, 88] subsequently decreasing safety [88] and increasing medication tolerance [71, 73].
The issue of which anti-TNFα was preferable was not resolved by a meta-analysis [84]. The analysis included 23 published studies, and synthesized data on the utility of TNFα as the first biologic for steroid and IMT-resistant pediatric chronic AU. The authors only included studies utilizing SUN-like outcome measures. They created pooled outcomes for “positive response to treatment” according to SUN as its primary outcome. Etanercept was inferior to infliximab and adalimumab. Although the point estimate of positive response to treatment for adalimumab was greater than that of infliximab, the difference was not statistically significant (87 %, 95 % CI 75–98 vs 72 %, 95 % CI 64–79). The authors acknowledge that limitations include limited applicability for non-JIA-U and the wide variation in infliximab dosing.
The comparative safety of the medications must be considered alongside their ability to control disease. Short-term adverse effects of TNF inhibition may include the development of cytopenias, serious infections, rashes, infusion reactions, and anaphylaxis. Few studies reported more than minor infusion reactions in children on infliximab, and, for the most part, these did not necessitate discontinuation of the medication [64–67, 69]. Infliximab is often co-administered with MTX, to limit HACA development, and it is possible that some of the minor adverse reactions, such as transient leukopenia and transaminitis, were due to MTX rather than infliximab. Notably, in the Italian cohorts, one patient in the Simonini et al. study (5.9 %) [86] and three in the Zannin et al. study (6.3 %) [85] developed infusion reactions that resulted in treatment discontinuation. These percentages are even lower than those shown in recent retrospective studies of children treated with infliximab (for all causes), in which 10–17 % developed infusion reactions, most of which were minor or moderate [89, 90]. Adalimumab was quite well tolerated, without serious adverse events [76, 79, 85]. Therefore, short-term adverse events were rare with either treatment.
There is an emerging body of literature suggesting that patients may achieve better uveitis control, or relief from side effects, by switching from one anti-TNFα to another. In one small series, five children with JIA-U switched from infliximab (5 mg/kg every 8 weeks for maintenance) to adalimumab. The four who switched for persistent uveitis achieved control on adalimumab [91]. The one who switched for worsening psoriasis had worsening joint disease on adalimumab and switched back to infliximab. In a meta-analysis of children who switched anti-TNFα for poor uveitis control, Simonini et al. [92] found that 75 % (30/40) were able to achieve control (95 % CI 51–100). Children switched from etanercept to adalimumab (11) or infliximab (6) or from infliximab to adalimumab (23), but no children switched from adalimumab to infliximab due to failure.
5.5 Predictors of Response to anti-TNFα
Despite the encouraging results with anti-TNFα for uveitis, not all patients achieve uveitis inactivity. While rheumatology and ophthalmology experts recommend that anti-TNFα be used as second-line agents, after tCS and MTX (or AZA) [37], there are multiple newer biologic options available or in development. It would be useful to have clinical decision tools that would help predict a patient’s likelihood of response to anti-TNFα. In a retrospective cohort that included 56 children, the rate of achievement of quiescence (≤0.5+ AC cell and on ≤2 gtt/day tCS) was 75 % (95 % CI 62–87) in 1 year [93]. Rate of quiescence was 20–25 % higher in patients with both JIA and AU. In contrast, when a sensitivity analysis was performed restricted to patients with JIA, the success rate was not altered from that of the entire cohort [86].
5.6 Safety of anti-TNFα
In addition to the short-term risk of infusion reactions, increased risk of malignancy and serious infection have been posited as theoretical long-term risks of anti-TNFα treatment. A meta-analysis of the use of anti-TNFα for RA suggested an increased risk of malignancy and serious infection following treatment, with a dose-dependent effect [94]. When other authors repeated this analysis, including data from an additional study, the odds ratios were lower, bringing into question whether the risk of malignancy was indeed increased [95]. However, in 2009, the FDA placed a Black Box warning on anti-TNFα because 48 cases of malignancies in children treated with anti-TNFα for JIA or inflammatory bowel disease had been reported through the Adverse Events Reporting System [96]. Subsequent database studies of adults with RA and children with JIA were unable to demonstrate an increased risk of cancer in individuals treated with anti-TNFα [97, 98]. Moreover, one population-based study in Sweden demonstrated a small but increased risk of malignancy in biologic-naïve children with JIA when compared with the general population, suggesting that the cancer risk might be associated with uncontrolled inflammation from disease rather than the treatment itself [99]. Similarly, studies in adults have not consistently demonstrated an increased risk of serious infections with anti-TNFα [100]. In a Medicaid-based study of children treated with anti-TNFα, the rates of hospitalization for serious infections were not increased in children treated with anti-TNFα for JIA relative to children with attention deficit hyperactivity disorder, although they were increased in children with JIA treated with high dose CS [101]. Overall, the data available suggest that anti-TNFα are relatively safe medications.
5.7 Summary of Anti-TNFα
In summary, anti-TNFα, both infliximab and adalimumab, have been extremely valuable and relatively safe additions to our clinical armamentarium to treat uveitis. Patients who have failed other treatment regimens are most often able to achieve control on anti-TNFα. Frequently, children are able to dramatically reduce or discontinue tCS. However, uveitis will reactivate in many of these children, and whether the effectiveness of one drug wanes over time more than the other remains controversial. Overall, anti-TNFα have spared children uveitis complications and cumulative doses of steroids.
A variety of short-term outcome measures should be considered in evaluating treatment success, including percent of patients who achieve inactive disease; rate of achievement of inactive disease; ability to ‘steroid-spare’; and maintenance of inactive disease in a ‘steroid-sparing’ fashion. Because studies have assessed different outcomes, inter-study comparisons are challenging. Even when looking at the same measure (e.g., percent who achieve control), studies have used different ways to score uveitis activity as well as different definitions of control; SUN nomenclature has helped with the latter, but developing consensus on the outcome measures to be used in research will be crucial for the former [102].
Other longer-term outcome measures may be even more clinically relevant. These include the number of complications and best visual acuity following treatment. Long-term follow-up studies to examine whether children treated with anti-TNF have better ocular outcomes than did children in the pre-TNF era have not yet been performed. Similarly, studies have not compared the long-term outcomes of children treated with different anti-TNF modalities. Such studies will be important in directing future therapy.
6 Blockade of Other Pro-Inflammatory Cytokines
6.1 Interference with Th17-Driven Inflammation
T-helper 17 (Th17) cells are important producers of pathogenic inflammatory cytokines. The Th17 pathway may be particularly important in uveitis, as numbers of Th17 cells rose in peripheral blood mononuclear cells during active uveitis in both people and in mice with experimental autoimmune uveoretinitis (EAU) [103]. A number of cytokines play an intertwining role in stimulating and maintaining Th17 activation, including interleukin-17 (IL-17), IL-6 and IL-12/23. In the following sections we discuss the therapeutic role of biologics that modulate the Th17 pathway.
6.1.1 IL-17 Blockade
AIN457 (secukinumab), a subcutaneous injectable that is now approved for psoriasis, is a human monoclonal that binds to and interferes with IL-17A signaling. IL-17A, an important driver of T cell-mediated inflammation, is produced by and stimulates Th17 CD4+ T cells. In the initial trial of secukinumab, patients with RA or psoriasis were treated with drug or placebo, and secukinumab was safe and effective [104]. In this trial, 16 adults with non-infectious uveitis (both AU and posterior uveitis) were treated with secukinumab but without a placebo-control group. Of these, 11 achieved decreased inflammation by 8 weeks. Three of five adult patients with AU achieved no inflammation (off topical or systemic CS). Subsequently, three larger placebo-controlled studies focusing on uveitis were initiated but ultimately were terminated early when the arm examining Behçet-associated posterior or pan-uveitis (SHIELD) failed to meet its primary endpoints. While phase III trials for its effectiveness for plaque psoriasis and its superiority to etanercept were reported in late 2014, there are no other reports of its use in uveitis [105]. Other monoclonal agents that interfere with IL-17 pathways are being developed, focusing on their effectiveness in psoriasis. There are no active clinical trials for its use in uveitis.
6.1.2 IL-6 Blockade
Tocilizumab is a humanized monoclonal antibody that binds to the IL-6-receptor, blocking IL-6 signaling. In experimental models of uveitis, IL-6, in conjunction with transforming growth factor (TGF)-β, has been important in both generating Th17 cells and in interfering with Treg development [106, 107]. Despite its theoretical utility, very little has been published on tocilizumab for uveitis in children. It has been shown to treat inflammatory eye disease in adults, including uveitis [108, 109]. Two case reports have described its benefit in maintaining quiescence in a total of four patients with recalcitrant JIA-U. Each had persistent activity despite CS, conventional immunosuppressants and TNF inhibitors (one had also failed abatacept and rituximab) [110, 111]. A clinical trial is currently underway to examine its utility.
6.1.3 IL-12/IL-23 Blockade
Ustekinumab is a human monoclonal antibody that interferes with IL-12/IL-23 signaling. It binds to the p40 subunit of free IL-12/IL-23 and interferes with their binding to membrane-bound receptors. Ustekinumab is FDA approved to treat moderate to severe plaque psoriasis. While there is a biologic rationale for IL-12/IL-23 blockade in uveitis, and the authors have seen good response in a child with severe psoriasis-associated uveitis (personal communication, MAL), there are no published reports of its use for uveitis. A clinical trial is being developed.
7 Interfering with IL-1 Signaling
A number of biologic agents exist that interfere with IL-1 signaling. The more commonly used agents for the pediatric age group include anakinra (analog of the natural IL-1 receptor antagonist) and canakinumab (monoclonal antibody to IL-1β). These are primarily used to treat systemic JIA, cryopyrinopathies, and macrophage activating syndrome. There is evidence that IL-1 signaling may play a role in uveitis. Lack of regulation of IL-1 signaling in a murine knockout of IL-1-receptor antagonist was associated with worse lipopolysaccharide (LPS)-induced uveitis than in wild-type mice [112]. Its blockade has been used for Behçet disease, which has a high incidence of uveitis, and there are case reports of its success in Behçet-associated uveitis [113, 114]. Gevokizumab (XOMA-052), an IL-1β-specific monoclonal antibody, is being actively studied for its use in adults, with and without Behçet disease, with non-anterior uveitis.
8 Inhibition of IL-2R Signaling
Daclizumab binds CD25 (IL-2 receptor-α) to block IL-2 receptor signaling. It was used primarily as a transplant drug, but also for lymphoma, graft vs host disease, multiple sclerosis, and uveitis. Although vision improved, uveitic flares were less frequent, and the need for IMT decreased in patients on daclizumab, it did not gain widespread use [115]. Production was halted in the US in 2009, although it is gaining momentum towards returning to market for multiple sclerosis.
9 Co-Stimulatory Blockade
Abatacept interferes with T-cell activation by inhibiting costimulation. T cells require two signals for activation. The first is an interaction between T-cell receptor (TCR) and its cognate antigen/MHC complex on the antigen-presenting cell (APC). The presence and type of the second signal impacts whether T cells are activated (CD28/B7.1 or B7.2), inhibited (CTLA4/B7), or anergized (no signal 2). Abatacept is a fusion protein between the extracellular portion of CTLA4 and the Fc of IgG1. It binds to B7 on the APC and limits the APC’s ability to provide continued stimulation to T cells, thereby dampening autoimmune responses. Abatacept was FDA approved in 2005 for treatment of MTX and TNF-inhibitor-resistant adult RA and is one of the few medications that can be administered either intravenously or subcutaneously (subcutaneous use approved for use in adults in 2011). In a randomized, double-blinded, placebo-controlled withdrawal design study of children with MTX-resistant or anti-TNF-resistant JIA, abatacept was shown to be safe, and at least partially effective, in decreasing joint disease activity [116]. Children with active uveitis were excluded.
While the effect on uveitis was not addressed in this large cohort, case reports are being published suggesting its effectiveness for JIA-U (Table 5). In 2008, Angeles-Han et al. [117] reported that abatacept (10 mg/kg) quieted uveitis in a child with recalcitrant psoriatic arthritis, IgA deficiency and uveitis whose uveitis had been resistant to conventional IMTs/immunosuppressive therapies (MTX, MMF, and CsA), CD25 inhibition, TNF inhibition, and B-cell depletion. While the child subsequently required continued low-dose prednisone (5 mg/day) and CsA, the uveitis remained quiet for 18 months of follow up. Abatacept resulted in decreased uveitis activity in 6/7 patients with severe bilateral JIA-U who had failed greater than two anti-TNFα [118]. The mean frequency of uveitis ‘flares’ (two degree increase in the level of AC cell) was decreased following treatment (3.7/6 months prior vs 0.7/6 months following). However, only two patients achieved full control (<1+ cell), and one of those remained on daily oral steroids (12.5 mg/day). Two more case series, each including two patients with JIA-U [119, 120] demonstrated abatacept’s utility in patients with anti-TNFα-resistant uveitis. In one case, cystoid macular edema resolved and vision improved (five Snellen lines). Interestingly, arthritis continued to be active in one patient whose uveitis was controlled [120].
In total, 12 patients treated with abatacept have been described. While it allowed systemic CS discontinuation in six of ten, tCS use was not addressed. Dosing regimens were similar. Most patients were treated with 10 mg/kg at weeks 0, 2, and then every 4 weeks thereafter. The time to achievement of uveitis control, albeit by varying definitions, ranged from 2 weeks to 6 months. Elhai et al. [119] described maintenance of uveitis control while spacing abatacept infusions to every 6 or 7 weeks, but attempts to decrease frequency of infusions have not been described by other groups. No studies have explored the durability of the uveitis response following abatacept withdrawal. However, in a murine EAU model, while costimulatory blockade limited T-cell activation, it did not result in long-term tolerance [121]. To date, there are no published data on subcutaneous abatacept for JIA-U. Neither descriptive studies nor randomized controlled trials have described the comparative effectiveness of abatacept as a second-line agent (after steroids and MTX) relative to TNF inhibitor. There is currently a clinical trial evaluating abatacept for uveitis.
10 B-Cell Depletion
Rituximab is a chimeric antibody to CD20, which is expressed on B lineage cells, except for pro-B cells and long-lived plasma cells in the bone marrow. It was FDA approved in 1997 to treat resistant lymphoma but since then was also approved to treat RA, granulomatosis with polyangiitis, and microscopic polyangiitis. Numerous small series have shown it to benefit patients with retinal vasculitis, keratitis, scleritis and orbital inflammatory disease, associated with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides, and Behçet disease [122, 123]. It has also been used with some success in JIA-U resistant to conventional IMTs and anti-TNFα (Table 6). In 2008, Angeles-Han et al. [117] described one patient whose uveitis was resistant to rituximab (and later responded to abatacept). Conversely, two larger retrospective case series reported benefit in children with active JIA-U resistant to CS, conventional IMTs, and anti-TNFα. Heiligenhaus et al. [124] treated ten females with JIA-U resistant to both topical and systemic CS, one or more IMT, and one or more anti-TNFα with rituximab (365 mg/m2 in two doses). Uveitis improved to <0.5+ AC in seven of ten patients within a mean of 3.1 months. It recurred in four patients (within a mean of 7.5 months), but improved with retreatment in three. The authors suggest that reactivation correlated with CD19 B-cell repletion. Both topical and systemic medications were decreased, although absolute final doses are not reported. All of the patients who responded had ANA+/RF−/HLA B27− oligoarticular JIA. Miserocchi et al. [122] reported that seven of eight Caucasian children with active ANA+ JIA-U, resistant to previous conventional IMTs and two to three anti-TNFα, achieved complete disease control following the addition of rituximab (1000 mg 2 weeks apart; with repeat infusions at month 12 and 21). Uveitis activity improved within 4–5 months of rituximab. The authors followed SUN criteria (but it is not clear if ‘no’ activity meant no cells or ≤0.5). All of the children were able to decrease their systemic steroids (4/6 remained on low-dose oral CS), tCS (most remained on 1–2 gtt/day), and to decrease their conventional IMT. In neither study did vision improve, but it stabilized in both. Therefore, there may be a role for rituximab in children who have failed other biologics. Rituximab is appealing in that it requires much less frequent dosing than anti-TNFα agents. Whether it might be more useful earlier in the treatment paradigm after tCS and MTX has not been studied.
11 Other Novel Therapeutics
The immune system can be manipulated in a myriad of ways, besides blocking pro-inflammatory cytokine signaling. Other new therapeutics that enhance anti-inflammatory cytokines (e.g., IL-10), block small molecule signaling (phosphodiesterase and tyrosine kinase inhibition), and interfere with lymphocyte adhesion and migration (e.g., blockade of the Sphingosine-1-phosphate receptor by fingolimod) [125] are in development, or in use, for other inflammatory indications. It is appealing that many are oral medications. Some are being explored for their potential to treat uveitis. For example, the Janus kinase inhibitor tofacitinib is an oral medication that was FDA approved in 2012 to treat RA. Although it is currently being studied to treat dry eyes, there are no published or registered trials for uveitis [126]. Apremilast, an oral inhibitor of phosphodiesterase 4, was FDA approved in 2014 for psoriatic arthritis. It is currently being tested for its use for other inflammatory diseases, including in Behçet disease. However, a safety and efficacy trial for uveitis was discontinued after apremilast was not effective in three patients.
12 Treatment Approaches
There are no set guidelines for treatment of pediatric AU, and treatment practices vary by experience and center. However, there are some treatment principles that experts generally agree on. Many experts are concerned that initiation of conventional IMT or biological therapy frequently occurs too late in children when practitioners are not comfortable with non-CS therapies. For AU, tCS is first-line therapy, and systemic corticosteroids are most often not needed, as long as there is no posterior segment involvement. Initiation of IMT should be considered in children who develop or have eye complications at first evaluation, as it has been shown that the presence of one complication (posterior synechia being the most common in JIA-AU) increases risk of a second complication [18]. IMT should also be considered when there is ongoing need for tCS beyond 2 drops a day for persistent uveitis (>3 months) [37]. MTX is the most common first non-steroid drug used in pediatric AU. Dosing can be oral or by subcutaneous injection, is weekly, but should be aggressively dosed. Doses up to 1 mg/kg, to a maximum of 25–30 mg weekly, are used by pediatric rheumatologists. Consideration of initiation of biologic therapy should be entertained in the setting of eye complications with severe inflammation, or partial or non-response to conventional IMT, or CS dependence >2 drops per day, or development of eye complications on IMT.
13 Conclusion
To date, physicians follow a sequential pathway of tCS, then conventional IMT (most often MTX), followed by anti-TNFα. Despite widespread use of biologic agents for uveitis by experts, lack of FDA indications for uveitis provides a considerable challenge as this translates into difficulty obtaining insurance coverage for these drugs. Furthermore, once patients are resistant to one anti-TNFα drug, there is little research to direct their subsequent choices between an alternate anti-TNFα (switching), or a biologic that targets a different pathway, such as abatacept, rituximab, or tocilizumab. In the future, biomarkers may help clinicians to better tailor their drug choices. Anti-TNFα agents have been on the market the longest, and are seen as the best options for MTX-resistant disease, but this may change as well. A recent study demonstrated that patients with TRAF-5 mutations are at increased risk of developing uveitis; these patients might benefit most from TNF blockade [127]. Conversely, in certain experimental models, and patients, intravitreous levels of Th17 cells and cytokines (such as IL-6) were elevated. If we could determine those individuals with Th17 predominant responses in the clinical setting, this would provide increased rationale to target the Th17 pathway. Unfortunately, local (intravitreous) and systemic cytokine profiling may not correlate. While we may be far from identifying biomarkers to predict response, studies in large cohorts may help identify whether the optimal agent depends on the underlying cause of the inflammation (e.g., JIA vs Behçet disease). What we do know is that after many years in which uveitis treatment options were quite limited, there are now numerous systemic options for treatment. We should now practice with the hope—and expectation—of the addition of new therapies, making this an exciting time for those who treat children with uveitis.
References
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–16.
Cunningham TE Jr. Uveitis in children. Ocul Immunol Inflamm. 2000;8(4):251–61.
Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112(7):1287–92.
Tugal-Tutkun I, Havrlikova K, Power WJ, Foster CS. Changing patterns in uveitis of childhood. Ophthalmology. 1996;103(3):375–83.
Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in english primary and referral centers. Am J Ophthalmol. 2003;135(5):676–80.
Sabri K, Saurenmann RK, Silverman ED, Levin AV. Course, complications, and outcome of juvenile arthritis-related uveitis. J AAPOS. 2008;12(6):539–45.
BenEzra D, Forrester JV, Nussenblatt RB, et al. Uveitis scoring system. Berlin: Springer; 1991. p. 1–9.
Foster CS, Vitale AT. Diagnosis and treatment of uveitis. Philadelphia: Saunders; 2002. p. 91–2.
Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. I. Anterior uveitis. Am J Ophthalmol. 1959;47:155–70.
Nussenblatt RB, Whitcup SM. Uveitis: fundamentals and clinical practice. Philadelphia: Mosby; 2004.
Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–71.
Paroli MP, Spinucci G, Fabiani C, Pivetti-Pezzi P. Retinal complications of juvenile idiopathic arthritis-related uveitis: a microperimetry and optical coherence tomography study. Ocul Immunol Inflamm. 2010;18(1):54–9.
Reiff A. Ocular complications of childhood rheumatic diseases: uveitis. Curr Rheumatol Rep. 2006;8(6):459–68.
Darrell RW, Wagener HP, Kurland LT. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol. 1962;68:502–14.
Päivönsalo-Hietanen T, Tuominen J, Saari KM. Uveitis in children: population-based study in Finland. Acta Ophthalmol Scand. 2000;78(1):84–8.
Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California epidemiology of uveitis study. Ophthalmology. 2004;111(3):491–500 (discussion 500).
Zierhut M, Michels H, Stübiger N, Besch D, Deuter C, Heiligenhaus A. Uveitis in children. Int Ophthalmol Clin. 2005;45(2):135–56.
Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K. German Uveitis in Childhood Study Group. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford). 2007;46(6):1015–9.
Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED. Risk factors for development of uveitis differ between girls and boys with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62(6):1824–8.
Kotaniemi K, Arkela-Kautiainen M, Haapasaari J, Leirisalo-Repo M. Uveitis in young adults with juvenile idiopathic arthritis: a clinical evaluation of 123 patients. Ann Rheum Dis. 2005;64(6):871–4.
Angeles-Han ST, Pelajo CF, Vogler LB, Rouster-Stevens K, Kennedy C, Ponder L, et al. Risk markers of juvenile idiopathic arthritis-associated uveitis in the childhood arthritis and rheumatology research alliance (CARRA) registry. J Rheumatol. 2013;40(12):2088–96.
Ravelli A, Felici E, Magni-Manzoni S, Pistorio A, Novarini C, Bozzola E, et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. 2005;52(3):826–32.
Saurenmann RK, Levin AV, Feldman BM, Rose JB, Laxer RM, Schneider R, Silverman ED. Prevalence, risk factors, and outcome of uveitis in juvenile idiopathic arthritis: a long-term followup study. Arthritis Rheum. 2007;56(2):647–57.
Cassidy J, Kivlin J, Lindsley C, Nocton J. Section on rheumatology, section on ophthalmology. Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 2006;117(5):1843–5.
Calandra S, Gallo MC, Consolaro A, Pistorio A, Lattanzi B, Bovis F, et al. Female sex and oligoarthritis category are not risk factors for uveitis in italian children with juvenile idiopathic arthritis. J Rheumatol. 2014;41(7):1416–25.
Chylack LT. The ocular manifestations of juvenile rheumatoid arthritis. Arthritis Rheum. 1977;20(Suppl 2):217–23.
Holland GN, Stiehm E. Special considerations in the evaluation and management of uveitis in children. Am J Ophthalmol. 2003;135(6):867–78.
Woreta F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;143(4):647–55.
Zulian F, Martini G, Falcini F, Gerloni V, Zannin ME, Pinello L, et al. Early predictors of severe course of uveitis in oligoarticular juvenile idiopathic arthritis. J Rheumatol. 2002;29(11):2446–53.
Edelsten C, Lee V, Bentley CR, Kanski JJ, Graham EM. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol. 2002;86(1):51.
Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143(5):840–6.
Holland GN. A reconsideration of anterior chamber flare and its clinical relevance for children with chronic anterior uveitis (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007;105:344–64.
Zannin ME, Buscain I, Vittadello F, Martini G, Alessio M, Orsoni JG, et al. Timing of uveitis onset in oligoarticular juvenile idiopathic arthritis (JIA) is the main predictor of severe course uveitis. Acta Ophthalmol. 2012;90(1):91–5.
Angeles-Han S, Yeh S. Prevention and management of cataracts in children with juvenile idiopathic arthritis-associated uveitis. Curr Rheumatol Rep. 2012;14(2):142–9.
Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010;117(7):1436–41.
Kazdan JJ, McCulloch JC, Crawford JS. Uveitis in children. Can Med Assoc J. 1967;96(7):385.
Heiligenhaus A, Michels H, Schumacher C, Kopp I, Neudorf U, Niehues T, et al. Evidence-based, interdisciplinary guidelines for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Rheumatol Int. 2012;32(5):1121–33.
Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513.
Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2005;32(2):362–5.
Gangaputra S, Newcomb CW, Liesegang TL, Kaçmaz RO, Jabs DA, Levy-Clarke GA, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116(11):2188–98.e1.
Heiligenhaus A, Mingels A, Heinz C, Ganser G. Methotrexate for uveitis associated with juvenile idiopathic arthritis: value and requirement for additional anti-inflammatory medication. Eur J Ophthalmol. 2007;17(5):743–8.
Malik AR, Pavesio C. The use of low dose methotrexate in children with chronic anterior and intermediate uveitis. Br J Ophthalmol. 2005;89(7):806–8.
Samson CM, Waheed N, Baltatzis S, Foster CS. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology. 2001;108(6):1134–9.
Weiss AW, Wallace CW, Sherry DS. Methotrexate for resistant chronic uveitis in children with juvenile rheumatoid arthritis. J Pediatr. 1998;133(133):266–8.
Doycheva D, Deuter C, Stuebiger N, Biester S, Zierhut M. Mycophenolate mofetil in the treatment of uveitis in children. Br J Ophthalmol. 2007;91(2):180–4.
Goebel JC, Roesel M, Heinz C, Michels H, Ganser G, Heiligenhaus A. Azathioprine as a treatment option for uveitis in patients with juvenile idiopathic arthritis. Br J Ophthalmol. 2011;95(2):209–13.
Daniel E, Thorne JE, Newcomb CW, Pujari SS, Kaçmaz RO, Levy-Clarke GA, et al. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010;149(3):423–32.e1-2.
Molina C, Modesto C, Martín-Begué N, Arnal C. Leflunomide, a valid and safe drug for the treatment of chronic anterior uveitis associated with juvenile idiopathic arthritis. Clin Rheumatol. 2013;32(11):1673–5.
BenEzra D, Cohen E, Rakotomalala M, de Courten C, Harris W, Chajek T, Friedman G, Matamoros N. Treatment of endogenous uveitis with cyclosporine A. Transplant Proc. 1988;20(3 Suppl 4):122–7.
Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117(3):576–84.
Kalinina Ayuso V, van de Winkel EL, Rothova A, DeBoer JH. Relapse rate of uveitis post-methotrexate treatment in juvenile idiopathic arthritis. Am J Ophthalmol. 2011;151(2):217–22.
Simonini G, Paudyal P, Jones GT, Cimaz R, Macfarlane GJ. Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach. Rheumatology (Oxford). 2013;52(5):825–31.
Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119(3):651–65.
Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by tnf-α in rheumatoid arthritis. Nat Med. 2013;19(3):322–8.
Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, Dewitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;63(4):465–82.
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(5):625–39.
Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU). Prog Retin Eye Res. 2004;23(6):617–37.
Santos Lacomba M, Marcos Martin C, Gallardo Galera JMA, Vidal G, Collantes Estevez E, Ramirez Chamond R, Omar MM. Aqueous humor and serum tumor necrosis factor-α in clinical uveitis. Ophthalmic Res. 2001;33(5):251–5.
Reiff A, Takei S, Sadeghi S, Stout A, Shaham B, Bernstein B, et al. Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum. 2001;44(6):1411–5.
Smith JR, Levinson RD, Holland GN, Jabs DA, Robinson MR, Whitcup SM, Rosenbaum JT. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum. 2001;45(3):252–7.
Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED. Risk of new-onset uveitis in patients with juvenile idiopathic arthritis treated with anti-tnfalpha agents. J Pediatr. 2006;149(6):833–6.
Smith JA, Thompson DJ, Whitcup SM, Suhler E, Clarke G, Smith S, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005;53(1):18–23.
Richards JC, Tay-Kearney ML, Murray K, Manners P. Infliximab for juvenile idiopathic arthritis-associated uveitis. Clin Experiment Ophthalmol. 2005;33(5):461–8.
Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006;113(2):308–14.
Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006;113(5):860–4.e2.
Sobrin L, Kim EC, Christen W, Papadaki T, Letko E, Foster CS. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol. 2007;125(7):895.
Ardoin SP, Kredich D, Rabinovich E, Schanberg LE, Jaffe GJ. Infliximab to treat chronic noninfectious uveitis in children: retrospective case series with long-term follow-up. Am J Ophthalmol. 2007;144(6):844–9.
Tugal-Tutkun I, Ayranci O, Kasapcopur O, Kir N. Retrospective analysis of children with uveitis treated with infliximab. J AAPOS. 2008;12(6):611–3.
Sharma SM, Ramanan AV, Riley P, Dick AD. Use of infliximab in juvenile onset rheumatological disease associated refractory uveitis: efficacy in joint and ocular disease. Ann Rheum Dis. 2007;66:840–1.
Simonini G, Zannin ME, Caputo R, Falcini F, de Martino M, Zulian F, Cimaz R. Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis. Rheumatology (Oxford). 2008;47(10):1510–4.
Ducourau E, Mulleman D, Paintaud G, Miow Lin DC, Lauféron F, Ternant D, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther. 2011;13(3):R105.
Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126(2):402–13.
Wolbink GJ, Vis M, Lems W, Voskuyl AE, de Groot E, Nurmohamed MT, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(3):711–5.
Vazquez-Cobian LB, Flynn T, Lehman TJA. Adalimumab therapy for childhood uveitis. J Pediatr. 2006;149(4):572–5.
Kempen JH, Ganesh SK, Sangwan VS, Rathinam SR. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol. 2008;146(6):813–8.e1.
Biester S, Deuter C, Michels H, Haefner R, Kuemmerle-Deschner J, Doycheva D, Zierhut M. Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol. 2007;91(3):319–24.
Bravo-Ljubetic L, Peralta-Calvo J, Noval S, Pastora-Salvador N, Abelairas-Gómez J, Merino R. Adalimumab therapy for refractory childhood uveitis. J AAPOS. 2013;17(5):456–9.
Gallagher M, Quinones K, Cervantes-Castaneda RA, Yilmaz T, Foster CS. Biological response modifier therapy for refractory childhood uveitis. Br J Ophthlamol. 2007;91(10):1341.
Tynjälä P, Kotaniemi K, Lindahl P, Latva K, Aalto K, Honkanen V, Lahdenne P. Adalimumab in juvenile idiopathic arthritis-associated chronic anterior uveitis. Rheumatology (Oxford). 2008;47(3):339–44.
Kotaniemi K, Säilä H, Kautiainen H. Long-term efficacy of adalimumab in the treatment of uveitis associated with juvenile idiopathic arthritis. Clin Ophthalmol. 2011;5:1425–9.
Sen ES, Sharma S, Hinchcliffe A, Dick AD, Ramanan AV. Use of adalimumab in refractory non-infectious childhood chronic uveitis: efficacy in ocular disease—a case cohort interventional study. Rheumatology (Oxford). 2012;51(12):2199–203.
Doycheva D, Zierhut M, Blumenstock G, Stuebiger N, Januschowski K, Voykov B, Deuter C. Immunomodulatory therapy with tumour necrosis factor α inhibitors in children with antinuclear antibody-associated chronic anterior uveitis: long-term results. Br J Ophthalmol. 2014;98(4):523–8.
Saurenmann RK, Levin AV, Rose JB, Parker S, Rabinovitch T, Tyrrell PN, et al. Tumour necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology (Oxford). 2006;45(8):982–9.
Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT. Current evidence of anti-tumor necrosis factor α treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs. Arthritis Care Res (Hoboken). 2014;66(7):1073–84.
Zannin ME, Birolo C, Gerloni VM, Miserocchi E, Pontikaki I, Paroli MP, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the Italian registry. J Rheumatol. 2013;40(1):74–9.
Simonini G, Taddio A, Cattalini M, Caputo R, De Libero C, Naviglio S, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res (Hoboken). 2011;63(4):612–8.
Sukumaran S, Marzan K, Shaham B, Reiff A. High dose infliximab in the treatment of refractory uveitis: does dose matter? ISRN Rheumatol. 2012;2012:765380.
Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56(9):3096–106.
Aeschlimann FA, Hofer KD, Cannizzaro Schneider E, Schroeder S, Lauener R, Saurenmann RK. Infliximab in pediatric rheumatology patients: a retrospective analysis of infusion reactions and severe adverse events during 2246 infusions over 12 years. J Rheumatol. 2014;41(7):1409–15.
Tambralli A, Beukelman T, Weiser P, Atkinson TP, Cron RQ, Stoll ML. High doses of infliximab in the management of juvenile idiopathic arthritis. J Rheumatol. 2013;40(10):1749–55.
Dhingra N, Morgan J, Dick AD. Switching biologic agents for uveitis. Eye (Lond). 2009;23(9):1868–70.
Simonini G, Katie D, Cimaz R, Macfarlane GJ, Jones GT. Does switching anti-tnfα biologic agents represent an effective option in childhood chronic uveitis: the evidence from a systematic review and meta-analysis approach. Semin Arthritis Rheum. 2014;44(1):39–46.
Lerman MA, Burnham JM, Chang PY, Daniel E, Foster CS, Hennessy S, et al. Response of pediatric uveitis to tumor necrosis factor-α inhibitors. J Rheumatol. 2013;40(8):1394–403.
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–85.
Costenbader KH, Glass R, Cui J, Shadick N. Risk of serious infections and malignancies with anti-TNF antibody therapy in rheumatoid arthritis. JAMA. 2006;296(18):2201.
Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the food and drug administration. Arthritis Rheum. 2010;62(8):2517–24.
Askling J, van Vollenhoven RF, Granath F, Raaschou P, Fored CM, Baecklund E, et al. Cancer risk in patients with rheumatoid arthritis treated with anti-tumor necrosis factor alpha therapies: does the risk change with the time since start of treatment? Arthritis Rheum. 2009;60(11):3180–9.
Beukelman T, Haynes K, Curtis JR, Xie F, Chen L, Bemrich-Stolz CJ, et al. Rates of malignancy associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum. 2012;64(4):1263–71.
Simard JF, Neovius M, Hagelberg S, Askling J. Juvenile idiopathic arthritis and risk of cancer: a nationwide cohort study. Arthritis Rheum. 2010;62(12):3776–82.
Schneeweiss S, Korzenik J, Solomon DH, Canning C, Lee J, Bressler B. Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther. 2009;30(3):253–64.
Beukelman T, Xie F, Chen L, Baddley JW, Delzell E, Grijalva CG, et al. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum. 2012;64(8):2773–80.
Heiligenhaus A, Foeldvari I, Edelsten C, Smith JR, Saurenmann RK, Bodaghi B, et al. Proposed outcome measures for prospective clinical trials in juvenile idiopathic arthritis-associated uveitis: a consensus effort from the multinational interdisciplinary working group for uveitis in childhood. Arthritis Care Res (Hoboken). 2012;64(9):1365–72.
Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–8.
Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17a, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8.
Mima T, Nishimoto N. Clinical value of blocking IL-6 receptor. Curr Opin Rheumatol. 2009;21(3):224–30.
Calvo-Río V, de la Hera D, Beltrán-Catalán E, Blanco R, Hernandez M, Martínez-Costa L, et al. Tocilizumab in uveitis refractory to other biologic drugs: a study of 3 cases and a literature review. Clin Exp Rheumatol. 2014;32(4 Suppl 84):S54–7.
Muselier A, Bielefeld P, Bidot S, Vinit J, Besancenot JF, Bron A. Efficacy of tocilizumab in two patients with anti-tnf-alpha refractory uveitis. Ocul Immunol Inflamm. 2011;19(5):382–3.
Tappeiner C, Heinz C, Ganser G, Heiligenhaus A. Is tocilizumab an effective option for treatment of refractory uveitis associated with juvenile idiopathic arthritis? J Rheumatol. 2012;39(6):1294–5.
Tsang AC, Roth J, Gottlieb C. Tocilizumab for severe chronic anterior uveitis associated with juvenile idiopathic arthritis in a pediatric patient. Ocul Immunol Inflam. 2013;22(2):155–7.
Planck SR, Woods A, Clowers JS, Nicklin MJ, Rosenbaum JT, Rosenzweig HL. Impact of IL-1 signalling on experimental uveitis and arthritis. Ann Rheum Dis. 2012;71(5):753–60.
Simonini G, Xu Z, Caputo R, De Libero C, Pagnini I, Pascual V, Cimaz R. Clinical and transcriptional response to the long-acting interleukin-1 blocker canakinumab in blau syndrome-related uveitis. Arthritis Rheum. 2013;65(2):513–8.
Ugurlu S, Ucar D, Seyahi E, Hatemi G, Yurdakul S. Canakinumab in a patient with juvenile Behcet’s syndrome with refractory eye disease. Ann Rheum Dis. 2012;71(9):1589–91.
Wroblewski K, Sen HN, Yeh S, Faia L, Li Z, Sran P, et al. Long-term daclizumab therapy for the treatment of noninfectious ocular inflammatory disease. Can J Ophthalmol. 2011;46(4):322–8.
Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372(9636):383–91.
Angeles-Han S, Flynn T, Lehman T. Abatacept for refractory juvenile idiopathic arthritis-associated uveitis—a case report. J Rheumatol. 2008;35(9):1897–8.
Zulian F, Balzarin M, Falcini F, Martini G, Alessio M, Cimaz R, et al. Abatacept for severe anti-tumor necrosis factor alpha refractory juvenile idiopathic arthritis-related uveitis. Arthritis Care Res (Hoboken). 2010;62(6):821–5.
Elhai M, Deslandre CJ, Kahan A. Abatacept for refractory juvenile idiopathic arthritis-associated uveitis: two new cases. Comment on the article by Zulian et al. Arthritis Care Res (Hoboken). 2011;63(2):307–8.
Kenawy N, Cleary G, Mewar D, Beare N, Chandna A, Pearce I. Abatacept: a potential therapy in refractory cases of juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;249(2):297–300.
Silver PB, Hathcock KS, Chan CC, Wiggert B, Caspi RR. Blockade of costimulation through B7/CD28 inhibits experimental autoimmune uveoretinitis, but does not induce long-term tolerance. J Immunol. 2000;165(9):5041–7.
Miserocchi E, Pontikaki I, Modorati G, Gattinara M, Meroni PL, Gerloni V. Anti-CD 20 monoclonal antibody (rituximab) treatment for inflammatory ocular diseases. Autoimmun Rev. 2011;11(1):35–9.
Pelegrin L, Jakob E, Schmidt-Bacher A, Schwenger V, Becker M, Max R, et al. Experiences with rituximab for the treatment of autoimmune diseases with ocular involvement. J Rheumatol. 2014;41(1):84–90.
Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-cd20 monoclonal antibody (rituximab). Rheumatology (Oxford). 2011;50(8):1390–4.
Commodaro AG, Peron JP, Lopes CT, Arslanian C, Belfort R, Rizzo LV, Bueno V. Evaluation of experimental autoimmune uveitis in mice treated with FTY720. Invest Ophthalmol Vis Sci. 2010;51(5):2568–74.
Stevenson W, Sadrai Z, Hua J, Kodati S, Huang JF, Chauhan SK, Dana R. Effects of topical janus kinase inhibition on ocular surface inflammation and immunity. Cornea. 2014;33(2):177–83.
Xiang Q, Chen L, Fang J, Hou S, Wei L, Bai L, et al. TNF receptor-associated factor 5 gene confers genetic predisposition to acute anterior uveitis and pediatric uveitis. Arthritis Res Ther. 2013;15(5):R113.
Reiff A. Long-term outcome of etanercept therapy in children with treatment-refractory uveitis. Arthritis Rheum. 2003;48(7):2079–80.
Acknowledgments
MA Lerman and CE Rabinovich declare no conflicts of interest. No sources of funding were used to support the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lerman, M.A., Rabinovich, C.E. The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis. Pediatr Drugs 17, 283–301 (2015). https://doi.org/10.1007/s40272-015-0128-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-015-0128-2