Abstract
Pasireotide (Signifor®) long-acting release (LAR) is a next-generation somatostatin receptor ligand (SRL) approved for treatment of patients with acromegaly who have had an inadequate response to surgery or for whom surgery is not an option. Pasireotide LAR has been shown to be more effective than other SRLs in providing biochemical control in patients with acromegaly. However, hyperglycemia-related adverse events were more frequent in patients treated with pasireotide LAR than in those treated with other SRLs. Given the effectiveness of pasireotide LAR, it is important to understand whether these hyperglycemia-related events are manageable and, if so, the appropriate steps to take to manage them. In patients treated with pasireotide LAR, levels of fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) increased in the first 1–3 months and stabilized for as long as 26 months thereafter. In phase III trials of patients with acromegaly, only 3.4–3.8 % discontinued pasireotide LAR because of hyperglycemia-related adverse events. In cases in which pasireotide LAR was discontinued, FPG and HbA1c levels returned to baseline. Frequent monitoring of glucose levels is recommended, especially immediately after initiating and discontinuing pasireotide LAR. The treatment strategies suggested herein are made on the basis of available clinical data from healthy volunteers and post hoc analyses of phase III trials. Data from several clinical trials indicate a predictable and possibly reversible hyperglycemic effect that is manageable with proactive monitoring and available antidiabetic medications.

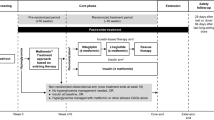
(Adapted with permission from [36]

(Adapted with permission from [39]

(Adapted with permission from [34]

Similar content being viewed by others
References
Ben-Shlomo A, Melmed S. Acromegaly. Endocrinol Metab Clin North Am. 2008;37(1):101–22, viii.
Lugo G, Pena L, Cordido F. Clinical manifestations and diagnosis of acromegaly. Int J Endocrinol. 2012;2012:540398.
Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159(2):89–95.
Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, et al. Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10(4):243–8.
Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update. Endocr Pract. 2011;17(suppl 4):1–44.
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–51.
Signifor LAR [package insert]. East Hanover: Novartis Pharmaceuticals Corporation; 2014.
Colao A, Bronstein MD, Freda P, Gu F, Shen CC, Gadelha M, et al. Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab. 2014;99(3):791–9.
Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875–84.
Signifor LAR [summary of product characteristics]. Basel: Novartis Pharma AG; 2014.
Colao A, De Block C, Gaztambide MS, Kumar S, Seufert J, Casanueva FF. Managing hyperglycemia in patients with Cushing’s disease treated with pasireotide: medical expert recommendations. Pituitary. 2014;17(2):180–6.
Reznik Y, Bertherat J, Borson-Chazot F, Brue T, Chanson P, Cortet-Rudelli C, et al. Management of hyperglycaemia in Cushing’s disease: experts’ proposals on the use of pasireotide. Diabetes Metab. 2013;39(1):34–41.
Golkowski F, Krzentowska-Korek A, Baldys-Waligorska A, Hubalewska-Dydejczyk A. Goiter, cardiovascular and metabolic disorders in patients with acromegaly. Endocr Regul. 2011;45(4):191–7.
Serri O, Beauregard C, Hardy J. Long-term biochemical status and disease-related morbidity in 53 postoperative patients with acromegaly. J Clin Endocrinol Metab. 2004;89(2):658–61.
Alexopoulou O, Bex M, Kamenicky P, Mvoula AB, Chanson P, Maiter D. Prevalence and risk factors of impaired glucose tolerance and diabetes mellitus at diagnosis of acromegaly: a study in 148 patients. Pituitary. 2014;17(1):81–9.
Kasayama S, Otsuki M, Takagi M, Saito H, Sumitani S, Kouhara H, et al. Impaired beta-cell function in the presence of reduced insulin sensitivity determines glucose tolerance status in acromegalic patients. Clin Endocrinol. 2000;52(5):549–55.
Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–77.
Moller N, Jorgensen JO, Alberti KG, Flyvbjerg A, Schmitz O. Short-term effects of growth hormone on fuel oxidation and regional substrate metabolism in normal man. J Clin Endocrinol Metab. 1990;70(4):1179–86.
Chen Y, Sun D, Krishnamurthy VM, Rabkin R. Endotoxin attenuates growth hormone-induced hepatic insulin-like growth factor I expression by inhibiting JAK2/STAT5 signal transduction and STAT5b DNA binding. Am J Physiol Endocrinol Metab. 2007;292(6):E1856–62.
Clemmons DR. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest. 2004;113(1):25–7.
Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41(2):425–43, vii-viii.
Burks DJ, White MF. IRS proteins and beta-cell function. Diabetes. 2001;50(Suppl 1):S140–5.
Boulware SD, Tamborlane WV, Rennert NJ, Gesundheit N, Sherwin RS. Comparison of the metabolic effects of recombinant human insulin-like growth factor-I and insulin. Dose–response relationships in healthy young and middle-aged adults. J Clin Invest. 1994;93(3):1131–9.
O’Connell T, Clemmons DR. IGF-I/IGF-binding protein-3 combination improves insulin resistance by GH-dependent and independent mechanisms. J Clin Endocrinol Metab. 2002;87(9):4356–60.
Ghigo E, Biller BM, Colao A, Kourides IA, Rajicic N, Hutson RK, et al. Comparison of pegvisomant and long-acting octreotide in patients with acromegaly naive to radiation and medical therapy. J Endocrinol Invest. 2009;32(11):924–33.
Bajetta E, Procopio G, Catena L, Martinetti A, De Dosso S, Ricci S, et al. Lanreotide Autogel every 6 weeks compared with Lanreotide microparticles every 3 weeks in patients with well differentiated neuroendocrine tumors: a phase III study. Cancer. 2006;107(10):2474–81.
Singh V, Brendel MD, Zacharias S, Mergler S, Jahr H, Wiedenmann B, et al. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. J Clin Endocrinol Metabol. 2007;92(2):673–80.
Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146(5):707–16.
Atiya AW, Moldovan S, Adrian TE, Coy D, Walsh J, Brunicardi FC. Intraislet somatostatin inhibits insulin (via a subtype-2 somatostatin receptor) but not islet amyloid polypeptide secretion in the isolated perfused human pancreas. J Gastrointest Surg. 1997;1(3):251–6 (discussion 6).
Tzanela M, Vassiliadi DA, Gavalas N, Szabo A, Margelou E, Valatsou A, et al. Glucose homeostasis in patients with acromegaly treated with surgery or somatostatin analogues. Clin Endocrinol. 2011;75(1):96–102.
Mazziotti G, Floriani I, Bonadonna S, Torri V, Chanson P, Giustina A. Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. J Clin Endocrinol Metab. 2009;94(5):1500–8.
Schmid HA, Brueggen J. Effects of somatostatin analogs on glucose homeostasis in rats. J Endocrinol. 2012;212(1):49–60.
Ludvigsen E, Stridsberg M, Taylor JE, Culler MD, Oberg K, Janson ET, et al. Regulation of insulin and glucagon secretion from rat pancreatic islets in vitro by somatostatin analogues. Regul Pept. 2007;138(1):1–9.
Sheppard M, Bronstein MD, Freda P, Serri O, De Marinis L, Naves L, et al. Pasireotide LAR maintains inhibition of GH and IGF-1 in patients with acromegaly for up to 25 months: results from the blinded extension phase of a randomized, double-blind, multicenter, phase III study. Pituitary. 2015;18(3):385–94.
Schmid HA, Brue T, Colao A, Gadelha MR, Shimon I, Kapur K, et al. Effect of pasireotide on glucose- and growth hormone-related biomarkers in patients with inadequately controlled acromegaly. Endocrine. 2016;53(1):210–9.
Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab. 2013;98(8):3446–53.
Plockinger U, Holst JJ, Messerschmidt D, Hopfenmuller W, Quabbe HJ. Octreotide suppresses the incretin glucagon-like peptide (7–36) amide in patients with acromegaly or clinically nonfunctioning pituitary tumors and in healthy subjects. Eur J Endocrinol. 1999;140(6):538–44.
Hansen L, Hartmann B, Bisgaard T, Mineo H, Jorgensen PN, Holst JJ. Somatostatin restrains the secretion of glucagon-like peptide-1 and -2 from isolated perfused porcine ileum. Am J Physiol Endocrinol Metab. 2000;278(6):E1010–8.
Breitschaft A, Hu K, Hermosillo Resendiz K, Darstein C, Golor G. Management of hyperglycemia associated with pasireotide (SOM230): healthy volunteer study. Diabetes Res Clin Pract. 2014;103(3):458–65.
Colao A, Gu F, Gadelha MR, van der Lely AJ, Fleseriu M, Passos VQ, et al. Metformin-based oral antidiabetic therapy is effective at controlling hyperglycemia associated with pasireotide in patients with acromegaly. Poster presented at: 97th Endocrine Society Annual Meeting and Expo; March 5–8, 2015; San Diego, CA.
Wildemberg LE, Gadelha MR. Pasireotide for the treatment of acromegaly. Expert Opin Pharmacother. 2016;17(4):579–88.
Fleseriu, M, Rusch E, Warsi G, Geer EB, on behalf of the ACCESS study investigators. Safety and tolerability of pasireotide long-acting release in acromegaly–results from the acromegaly, open-label, multi-center, safety monitoring program for treating patients who have need to receive medical therapy (ACCESS) study. Poster presented at: 97th Endocrine Society Annual Meeting and Expo; April 1–4, 2016; Boston, MA.
Lu L, Duan L, Jin Z, Lu Z, Gu F. Effective long-term treatment of Cushing’s disease with pasireotide: a case report. Endocr Prac. 2013;19(4):e92–6.
MacKenzie Feder J, Bourdeau I, Vallette S, Beauregard H, Ste-Marie LG, Lacroix A. Pasireotide monotherapy in Cushing’s disease: a single-centre experience with 5-year extension of phase III trial. Pituitary. 2014;17(6):519–29.
Freda P, Fleseriu M, van der Lely AJ, Colao A, Sheppard A, Gu F et al. Switching patients with acromegaly from octreotide LAR to pasireotide LAR improves biochemical control: crossover extension to a randomized, double-blind, multicenter, phase III study. Poster presented at: 15th European Congress of Endocrinology; April 27–May 1, 2013; Copenhagen, Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Editorial assistance under the author’s direction was provided by MedThink SciCom and was supported by Novartis Pharmaceuticals Corporation.
Conflict of interest
Susan L. Samson is a member of the pasireotide study group and has been a site principal investigator for SOM230 clinical trials involving acromegaly and Cushing’s disease. She is the recipient of an investigator-initiated grant on pasireotide effects on β-cell function and replication. She also is a steering committee member for the ongoing SOM230B2219 trial.
Rights and permissions
About this article
Cite this article
Samson, S.L. Management of Hyperglycemia in Patients With Acromegaly Treated With Pasireotide LAR. Drugs 76, 1235–1243 (2016). https://doi.org/10.1007/s40265-016-0615-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0615-y