Abstract
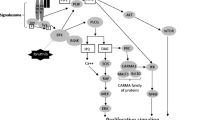
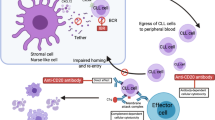
Ibrutinib (Imbruvica®) is a first-in-class, potent, orally administered, covalent inhibitor of Bruton’s tyrosine kinase (BTK) that inhibits B-cell antigen receptor signalling downstream of BTK. Oral ibrutinib is indicated for the treatment of patients with relapsed/refractory mantle cell lymphoma (MCL) or chronic lymphocytic leukaemia (CLL) and for the treatment of patients with CLL and a chromosome 17 deletion (del 17p) or TP53 mutation. This article summarizes pharmacological, efficacy and tolerability data relevant to the use of ibrutinib in these indications. In clinical studies, ibrutinib induced a high overall response rate in patients with relapsed/refractory MCL (phase II study). In addition, ibrutinib significantly prolonged progression-free survival and significantly improved the partial response rate and overall survival in patients with relapsed/refractory CLL (RESONATE study), including in those with del 17p, a subgroup with a poor prognosis. Ibrutinib had an acceptable tolerability profile in these studies with <10 % of patients discontinuing treatment because of adverse events. Given its efficacy and tolerability, once-daily, oral ibrutinib is an emerging treatment option for patients with relapsed/refractory MCL or CLL and CLL patients with del 17p or TP53 mutation.

Similar content being viewed by others
References
Miranda RN, Khoury JD, Medeiros LJ. Mantle cell lymphoma. In: Miranda RN, Khoury JD, Medeiros LJ, editors. Atlas of lymph node pathology. New York: Springer; 2013. p. 229–35.
Boelens J, Lust S, Vanhoecke B, et al. Chronic lymphocytic leukemia. Anticancer Res. 2009;29(2):605–15.
Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–8.
McKay P, Leach M, Jackson R, et al. Guidelines for the investigation and management of mantle cell lymphoma. Br J Haematol. 2012;159(4):405–26.
Dreyling M. Mantle cell lymphoma: biology, clinical presentation, and therapeutic approaches. 2014 educational book: American Society of Clinical Oncology; 2014. p. 191–8.
Wierda WG, O’Brien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109(11):4679–85.
Brown JR. Ibrutinib (PCI-32765), the first BTK (Bruton’s tyrosine kinase) inhibitor in clinical trials. Curr Hematol Malig Rep. 2013;8(1):1–6.
Sellner L, Denzinger S, Dietrich S, et al. What do we do with chronic lymphocytic leukemia with 17p deletion? Curr Hematol Malig Rep. 2013;8(1):81–90.
Eichhorst B, Dreyling M, Robak T, et al. Chronic lymphocytic leukemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi50–4.
Arnason JE, Brown JR. Targeted therapy for chronic lymphocytic leukemia: current status and future directions. Drugs. 2015;75(2):143–55.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) non-Hodgkin’s lymphomas. 2015. https://www.nccn.org. Accessed 3 Feb 2015.
Dreyling M, Geisler C, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii83–92.
Ghia P. Ibrutinib: better combined with other drugs? Lancet Oncol. 2014;15(10):1043–4.
Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest. 2012;122(10):3416–23.
Cheng S, Ma J, Guo A, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28(3):649–57.
Burger JA. Bruton’s tyrosine kinase (BTK) inhibitors in clinical trials. Curr Hematol Malig Rep. 2014;9(1):44–9.
Cinar M, Hamedani F, Mo Z, et al. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by ibrutinib induces apoptosis. Leuk Res. 2013;37(10):1271–7.
Herman SEM, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–96.
Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120(6):1175–84.
US FDA. Imbruvica (ibrutinib) capsules: US prescribing information. 2015. http://www.accessdata.fda.gov. Accessed 12 Mar 2015.
European Medicines Agency. Imbruvica (ibrutinib) hard capsules: EU summary of product characteristics. 2014. http://ec.europa.eu. Accessed 12 Mar 2015.
Cameron F, Sanford M. Ibrutinib: first global approval. Drugs. 2014;74(2):263–71.
Johnson and Johnson. Application submitted to the EMA to expand the therapeutic indication for Imbruvica® (ibrutinib) to include treatment of Waldenström’s macroglobulinemia. 2014. http://www.investor.jnj.com. Accessed 9 Feb 2015.
Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107(29):13075–80.
Herman SEM, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-kB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123(21):3286–95.
Chang BY, Francesco M, De Rooij MFM, et al. Egress of CD19+ CD5+ cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood. 2013;122(14):2412–24.
Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88–94.
Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42.
Herman SEM, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia. 2014;28(11):2188–96.
Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–23.
Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–16.
Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–7.
Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286–94.
Martin P, Maddocks KJ, Noto K, et al. Poor overall survival of patients with ibrutinib-resistant mantle cell lymphoma [abstract no. 3047]. In: 56th ASH Annual Meeting and Exposition; 2014.
Chiron D, Di Liberto M, Martin P, et al. Cell-cycle reprogramming for PI3 K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov. 2014;4(9):1022–35.
Wang M, Rule S, Martin P, et al. Single-agent ibrutinib demonstrates safety and durability of response at 2 years follow-up in patients with relapsed or refractory mantle cell lymphoma: updated results of an international, multicenter, open-label phase 2 study [abstract no. 4453]. Blood. 2014;124(21).
O’Brien S, Jones JA, Coutre S, et al. Efficacy and safety of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic leukemia with 17p deletion: results from the phase II RESONATE™-17 trial [abstract no. 327]. In: 56th ASH Annual Meeting and Exposition; 2014.
Brown JR, Hillmen P, O’Brien S, et al. Updated efficacy including genetic and clinical subgroup analysis and overall safety in the phase 3 RESONATE™ trial of ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma [abstract no. 3331]. Blood. 2014;124(21).
Barrientos JC, O’Brien S, Brown JR, et al. Hematologic and immunologic function and patient well-being for the phase III RESONATE™ study of ibrutinib vs ofatumumab in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma [abstract no. 4696]. In: 56th ASH Annual Meeting and Exposition; 2014.
McMullen JR, Boey EJH, Ooi JYY, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–30.
Byrd JC, Hillmen P, James DF. Response: additional data needed for a better understanding of the potential relationship between atrial fibrillation and ibrutinib. Blood. 2015;125(10):1673.
Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood. 2015;125(1):48–55.
Tongbram V, Sengupta N, Gaudig M, et al. Comparative effectiveness of treatments for relapsed or refractory mantle cell lymphoma (R/R MCL), using matching adjusted indirect comparison [abstract no. PCN5]. Value Health. 2014;17(7):A614–5.
Da Roit F, Engelberts PJ, Taylor RP, et al. Ibrutinib interferes with the cell-mediated anti-tumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica. 2015;100(1):77–86.
Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–9.
Wang M, Hagemeister F, Westin JR, et al. Ibrutinib and rituximab are an efficacious and safe combination in relapsed mantle cell lymphoma: preliminary results from a phase II clinical trial [abstract no. 627]. In: 56th ASH Annual Meeting and Exposition; 2014.
Hallek M, Kay NE, Osterborg A, et al. The HELIOS trial protocol: a phase III study of ibrutinib in combination with bendamustine and rituximab in relapsed/refractory chronic lymphocytic leukemia. Future Oncol. 2015;11(1):51–9.
Pan F, Peng S, Sorensen S, et al. Simulation model of ibrutinib for chronic lymphocytic leukemia (CLL) with prior treatment [abstract no. PCN40]. Value Health. 2014;17(7):A620–1.
Peng S, Sorensen S, Pan F, et al. Simulation model of ibrutinib in treatment of relapsed or refractory mantle cell lymphoma (MCL) [abstract no. PCN38]. Value Health. 2014;17(7):A620.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author(s) on the basis of scientific and editorial merit. Esther Kim and Sohita Dhillon are salaried employees of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: J. Delgado, Department of Hematology, Hospital Clinic, IDIBAPS, Barcelona, Spain; M.M. Dreyling, Department of Medicine III, University Hospital, Ludwig-Maximilians University, Munich, Germany; S. P. Mulligan, Royal North Shore Hospital, Sydney, NSW, Australia; N. Reddy, Department of Medicine, Division of Hematology/Oncology, Vanderbilt University Medical Center, Nashville, TN, USA.
Rights and permissions
About this article
Cite this article
Kim, E.S., Dhillon, S. Ibrutinib: A Review of Its Use in Patients with Mantle Cell Lymphoma or Chronic Lymphocytic Leukaemia. Drugs 75, 769–776 (2015). https://doi.org/10.1007/s40265-015-0380-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0380-3