Abstract
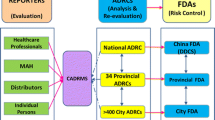
It has been more than 25 years since an adverse drug reaction (ADR) monitoring agency was first established in China. In the past few years, the National ADR Monitoring System (NADRMS) has developed rapidly in the country. However, this system has not been reviewed in detail in the literature. Our aim was to demonstrate how individual case safety reports (ICSRs) are reported and evaluated and how data quality control is achieved in China. We also aimed to discuss the present status and future of NADRMS. We reviewed the relevant regulations and literature around ADR reporting in China. ADR report collection tools in China have gone through three phases, namely paper-based reporting, software-based reporting using standalone computers, and online reporting. Nowadays the online reporting system plays an important role in China and the number of ADR reports has rapidly increased. NADRMS is similar to most of the ADR reporting systems around the world, but also has its own unique characteristics such as four levels of monitoring agencies. In summary, there is still a long way to go for China to establish a high-level ADR monitoring system to ensure drug safety.



Similar content being viewed by others
References
Du Xiaoxi, Zhang Chengxu, Guo Huipu, et al. Adverse drug reactions monitoring and supervision. Beijing: China Medical Science Press; 2013.
China Food and Drug Administration. Provisions for adverse drug reaction reporting and monitoring. 2004. http://www.sda.gov.cn/WS01/CL0053/24477.html. Accessed 19 July 2016.
Ministry of Health. Provisions for Adverse Drug Reaction Reporting and Monitoring. 2011. http://www.nhfpc.gov.cn/mohzcfgs/pgz/201105/51770.shtml. Accessed 19 July 2016.
China Food and Drug Administration. Provisions for drug registration. 2007. http://www.sda.gov.cn/WS01/CL0053/24529.html. Accessed 19 July 2016.
National Health and Family Planning Statistics Information Center. Number of medical institutions nationwide. 2015. http://www.nhfpc.gov.cn/mohwsbwstjxxzx/s7967/201501/1e39cfd2159d426c8e46e29280dbd7f9.shtml. Accessed 19 July 2016.
Hongyun Feng, Hou Yongfang, Wu Guizhi, Cheng Gang. The establishing and running of pre-warning system on adverse event cluster signals. Chin J Pharmacovigil. 2012;9(12):745–7.
A review of the methotrexate incident. 2007. http://finance.sina.com.cn/consume/puguangtai/20071213/07194285259.shtml. Accessed 19 July 2016.
A review of the Xinfu (Clindamycin Phosphate and Glucose Injection) incident. 2006. http://news.sohu.com/s2006/xinfuhuigu/. Accessed 19 July 2016.
The Armillarisin incident. 2006. http://news.sina.com.cn/z/qqhescjy/. Accessed 19 July 2016.
European medicines agency. Guideline on Detection and management of duplicate individual cases and individual case safety reports. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2010/06/WC500094013.pdf. Accessed 19 July 2016.
Norén GN, Orre R, Bate A, Edwards IR. Duplicate detection in adverse drug reaction surveillance. Data Min Knowl Discov. 2007;14(3):305–28.
Tregunno PM, Fink DB, Fernandez-Fernandez C, Lazaro-Bengoa E, Noren GN. Performance of probabilistic method to detect duplicate individual case safety reports. Drug Saf. 2014;37(4):249–58.
Yongfang Hou. The standardization in adverse drug reaction data. China Licens Pharm. 2010;7(4):13–5.
Acknowledgments
We would like to extend our special gratitude to Marie Lindquist and Zhurong Liu, from the Uppsala Monitoring Center for helpful comments on earlier versions of this paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this review.
Conflict of interest
Yongfang Hou, Xinling Li, Guizhi Wu, and Xiaofei Ye have no conflicts of interest that are directly relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Hou, Y., Li, X., Wu, G. et al. National ADR Monitoring System in China. Drug Saf 39, 1043–1051 (2016). https://doi.org/10.1007/s40264-016-0446-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0446-5