Abstract
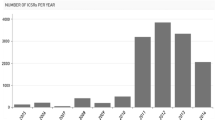
With the integration of the global pharmaceutical economy and the gradual transformation of the healthcare insurance system in China, the legislative framework for a comprehensive regulatory system monitoring the whole process including drug development, manufacture, distribution and use has been established by the China Food and Drug Administration (CFDA) to ensure the safety and effectiveness of medication use. China has established a relatively comprehensive pharmacovigilance system covering regulation, organisation and technology from 1989 to 2014. As of 2013, one national centre, 34 provincial centres and more than 400 municipal centres for adverse drug reaction (ADR) monitoring were included in the four-level pharmacovigilance network (national, provincial, municipal and county) with more than 200,000 grassroot organisation users. The China Adverse Drug Reaction Monitoring System (CADRMS) is an online spontaneous reporting system which connects the four-level pharmacovigilance network. By 2013, CADRMS had received over 6.6 million ADR case reports. After integrating and analysing pharmacovigilance data, the National Centre for ADR Monitoring (NCADRM) publishes medication safety information by releasing ADR bulletins, National ADR Annual Reports and International Pharmacovigilance Newsletters. The NCADRM also routinely provides CADRMS data feedback to manufacturers. The CFDA implemented risk management through several approaches, including arranging ‘manufacturer communication meetings’, modification of medication package inserts, and restriction, suspension or withdrawal of marketing authorisations. Seamless information exchange with overseas regulatory authorities and organisations remains an area for improvement. Further development of the China pharmacovigilance system in terms of signal generation, post-marketing pharmacoepidemiology research and education is also needed.

Similar content being viewed by others
References
National Bureau of Statistics of the People’s Republic of China, China Statistical Yearbook 2013. http://www.stats.gov.cn/tjsj/ndsj/2013/indexeh.htm. Accessed 21 Aug 2014.
Information Office of the State Council, The People’s Republic of China. Medical and health services in China (December 2012). http://www.china-embassy.org/eng/zt/bps/t1001641.htm. Accessed 14 Aug 2014.
Sun XZ. Take scientific and steady step to promote complete coverage of drug electronic supervision in China. Chin J Pharm Guide. 2012;12(3):369–71.
Zhang L, Yan J, Liu X, Ye Z, Yang X, Meyboom R, et al. Pharmacovigilance practice and risk control of traditional Chinese medicine drugs in China: current status and future perspective. J Ethnopharm. 2012;140(3):519–25.
CFDA Releases 2013 Annual Report for National ADR Monitoring. China Food and Drug Newsletter. 2014;5:2–5. http://www.ccpie.org/news/download/2014pharm-5.pdf. Accessed 4 Sept 2014.
China Food and Drug Administration. Annual ADR report on national ADR monitoring, May 2014. http://www.sda.gov.cn/WS01/CL0078/99794.html. Accessed 4 Sept 2014.
Ministry of Health and China Food and Drug Administration, Adverse drug reaction reporting and monitoring provision, May 2011. http://www.sfda.gov.cn/WS01/CL0053/62621.html. Accessed 21 Aug 2014.
Zhang L, Yang XH, Cao LY, et al. Pondering on current status and development of monitoring on adverse reaction of traditional Chinese medicine in China. Chin J Integr Tradit West Med. 2005;25(7):581–4.
Chen F, Liu S, Wu J. Puerarin-induced immune hemolytic anemia. Int J Hematol. 2013;98(1):112–3.
Lin BL, Zhao ZX, Chong YT, Li JG, Zuo X, Tao Y, et al. Venous diethylene glycol poisoning in patients with preexisting severe liver disease in China. World J Gastroenterol. 2008;14(20):3236–41.
The International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH). ICH Harmonised tripartite guideline pharmacovigilance planning E2E (18 Nov 2004). http://www.ich.org/products/guidelines/efficacy/efficacy%20single/article/pharmacovigilance-planning.html. Accessed 21 Aug 2014.
European Medicines Agency, Good pharmacovigilance practices. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000345.jsp. Accessed 21 Aug 2014.
International Society for Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practices (GPP) (Apr 2007). https://www.pharmacoepi.org/resources/guidelines_08027.cfm. Accessed 21 Aug 2014.
Declaration of funding and conflict of interest
No sources of funding were used to assist in the preparation of this study. Li Zhang, Lisa Y.L. Wong, Ying He and Ian C.K. Wong have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Wong, L.Y.L., He, Y. et al. Pharmacovigilance in China: Current Situation, Successes and Challenges. Drug Saf 37, 765–770 (2014). https://doi.org/10.1007/s40264-014-0222-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-014-0222-3