Abstract
The amyloid β-protein (Aβ) plays an indispensable role in the pathogenesis of Alzheimer disease (AD). Aβ is subject to proteolytic degradation by a diverse array of peptidases and proteinases, known collectively as Aβ-degrading proteases (AβDPs). A growing number of AβDPs have been identified that impact Aβ powerfully and in a surprising variety of ways. As such, AβDPs hold considerable therapeutic potential for the treatment and/or prevention of AD. Here, we critically review the relative merits of therapeutic strategies targeting AβDPs compared with current Aβ-lowering strategies focused on immunotherapies and pharmacological modulation of Aβ-producing enzymes. Several innovative advances have increased considerably the feasibility of delivering AβDPs to the brain or enhancing their activity in a non-invasive manner. We argue that therapies targeting AβDPs offer numerous potential advantages that should be explored through continued research into this promising field.
Similar content being viewed by others
References
Mucke L. Neuroscience: Alzheimer’s disease. Nature. 2009;461(7266):895–7.
Prince M, Wimo A, Guerchet M, Ali GC, Wu TT, Prina M, et al. World Alzheimer’s Report 2015. The global impact of dementia: an analysis of prevalence, incedence, cost and trends. London: Alzheimer’s Disease International; 2015.
Alzheimer’s A. 2015 Alzheimer’s disease facts and figures. Alzheimer Dement. 2015;11(3):332–84.
James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82(12):1045–50. doi:10.1212/WNL.0000000000000240.
Godyn J, Jonczyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep. 2016;68(1):127–38. doi:10.1016/j.pharep.2015.07.006.
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6. doi:10.1126/science.1072994.
Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–84.
Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harbor Perspect Med. 2012. doi:10.1101/cshperspect.a006296.
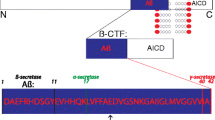
Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51(6):783–6. doi:10.1002/ana.10208.
Holsinger RM, Lee JS, Boyd A, Masters CL, Collins SJ. CSF BACE1 activity is increased in CJD and Alzheimer disease versus [corrected] other dementias. Neurology. 2006;67(4):710–2. doi:10.1212/01.wnl.0000229925.52203.4c.
Leissring MA. Proteolytic degradation of the amyloid beta-protein: the forgotten side of Alzheimer’s disease. Curr Alzheimer Res. 2006;3(5):431–5.
Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10(5):333–44. doi:10.1038/nrn2620.
Mawuenyega KG, Kasten T, Sigurdson W, Bateman RJ. Amyloid-beta isoform metabolism quantitation by stable isotope-labeled kinetics. Anal Biochem. 2013;440(1):56–62. doi:10.1016/j.ab.2013.04.031.
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. doi:10.1126/science.1197623.
De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6(2):99–107.
Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131(2):215–21.
Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13(2):159–70. doi:10.1093/hmg/ddh019.
Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L Jr, Eckman C, et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264(5163):1336–40.
Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, et al. BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time. Neurobiol Dis. 2003;14(1):81–8 (S0969996103001049 [pii]).
Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, et al. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4(3):231–2. doi:10.1038/85059.
Cole SL, Vassar R. BACE1 structure and function in health and Alzheimer’s disease. Curr Alzheimer Res. 2008;5(2):100–20.
Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, et al. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31(14):3157–68. doi:10.1038/emboj.2012.173.
Fleck D, van Bebber F, Colombo A, Galante C, Schwenk BM, Rabe L, et al. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J Neurosci. 2013;33(18):7856–69. doi:10.1523/JNEUROSCI.3372-12.2013.
Filser S, Ovsepian SV, Masana M, Blazquez-Llorca L, Brandt Elvang A, Volbracht C, et al. Pharmacological inhibition of BACE1 impairs synaptic plasticity and cognitive functions. Biol Psychiatry. 2015;77(8):729–39. doi:10.1016/j.biopsych.2014.10.013.
Yan R, Vassar R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014;13(3):319–29. doi:10.1016/S1474-4422(13)70276-X.
Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89(4):629–39.
Selkoe DJ. Presenilin, Notch, and the genesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98(20):11039–41.
Lleo A, Saura CA. gamma-secretase substrates and their implications for drug development in Alzheimer’s disease. Curr Top Med Chem. 2011;11(12):1513–27.
De Strooper B. Lessons from a failed gamma-secretase Alzheimer trial. Cell. 2014;159(4):721–6. doi:10.1016/j.cell.2014.10.016.
Siemers ER, Dean RA, Friedrich S, Ferguson-Sells L, Gonzales C, Farlow MR, et al. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-beta after inhibition of gamma-secretase. Clin Neuropharmacol. 2007;30(6):317–25. doi:10.1097/WNF.0b013e31805b7660.
Ascher-Svanum H, Chen YF, Hake A, Kahle-Wrobleski K, Schuster D, Kendall D, et al. Cognitive and functional decline in patients with mild alzheimer dementia with or without comorbid diabetes. Clin Ther. 2015;37(6):1195–205. doi:10.1016/j.clinthera.2015.01.002.
Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, et al. An atomic structure of human gamma-secretase. Nature. 2015;525(7568):212–7. doi:10.1038/nature14892.
Wolfe MS. Inhibition and modulation of gamma-secretase for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):391–8.
Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112(3):440–9. doi:10.1172/JCI18162.
De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and gamma-secretase: structure, function, and role in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(1):a006304. doi:10.1101/cshperspect.a006304.
Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–9.
DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98(15):8850–5.
Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, et al. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002;8(11):1270–5.
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–7.
Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, et al. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann Neurol. 2000;48(4):567–79.
Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–23. doi:10.1016/S0140-6736(08)61075-2.
Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38(4):547–54.
Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9(4):448–52.
Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ. Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol. 2012;8(8):465–9. doi:10.1038/nrneurol.2012.118.
Fuller JP, Stavenhagen JB, Teeling JL. New roles for Fc receptors in neurodegeneration—the impact on immunotherapy for Alzheimer’s disease. Front Neurosci. 2014;8:235. doi:10.3389/fnins.2014.00235.
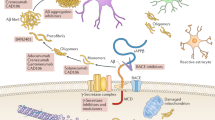
Eckman EA, Adams SK, Troendle FJ, Stodola BA, Kahn MA, Fauq AH, et al. Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem. 2006;281(41):30471–8.
Eckman EA, Reed DK, Eckman CB. Degradation of the Alzheimer’s amyloid beta peptide by endothelin-converting enzyme. J Biol Chem. 2001;276(27):24540–8.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457–70. doi:10.1038/nrneurol.2015.119.
Leissring MA, Saido TC. Degradation of amyloid-β protein. In: Selkoe DJ, Mandelkow E, Holtzman DM, editors. The biology of Alzheimer disease. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2011. p. 387–404.
Saido T, Leissring MA. Proteolytic degradation of amyloid β-protein. Cold Spring Harb Perspect Med. 2012;2(6):a006379. doi:10.1101/cshperspect.a006379.
Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron. 2006;51(6):703–14. doi:10.1016/j.neuron.2006.07.027.
Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11(3):213–28.
Shankar GM, Leissring MA, Adame A, Sun X, Spooner E, Masliah E, et al. Biochemical and immunohistochemical analysis of an Alzheimer’s disease mouse model reveals the presence of multiple cerebral Abeta assembly forms throughout life. Neurobiol Dis. 2009;36(2):293–302.
Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40(6):1087–93.
Meilandt WJ, Cisse M, Ho K, Wu T, Esposito LA, Scearce-Levie K, et al. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci. 2009;29(7):1977–86. doi:10.1523/JNEUROSCI.2984-08.2009.
Leissring MA, Turner AJ. Regulation of distinct pools of amyloid beta-protein by multiple cellular proteases. Alzheimer Res Ther. 2013;5(4):37. doi:10.1186/alzrt194.
LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8(7):499–509.
Leissring MA. Aβ degradation-the inside story. Front Aging Neurosci. 2014;6:229. doi:10.3389/fnagi.2014.00229.
Lewis PA, Piper S, Baker M, Onstead L, Murphy MP, Hardy J, et al. Expression of BRI-amyloid beta peptide fusion proteins: a novel method for specific high-level expression of amyloid beta peptides. Biochim Biophys Acta. 2001;1537(1):58–62.
Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, et al. Abeta40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27(3):627–33. doi:10.1523/JNEUROSCI.4849-06.2007.
Kim J, Chakrabarty P, Hanna A, March A, Dickson DW, Borchelt DR, et al. Normal cognition in transgenic BRI2-Abeta mice. Mol Neurodegener. 2013;8:15. doi:10.1186/1750-1326-8-15.
Leissring MA. The AβCs of Aβ-cleaving proteases. J Biol Chem. 2008;283(44):29645–9.
Matsuoka Y, Saito M, LaFrancois J, Saito M, Gaynor K, Olm V, et al. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23(1):29–33.
Liu Y, Studzinski C, Beckett T, Guan H, Hersh MA, Murphy MP, et al. Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease. Mol Ther. 2009;17(8):1381–6. doi:10.1038/mt.2009.115.
Liu Y, Studzinski C, Beckett T, Murphy MP, Klein RL, Hersh LB. Circulating neprilysin clears brain amyloid. Mol Cell Neurosci. 2010;45(2):101–7. doi:10.1016/j.mcn.2010.05.014.
Henderson SJ, Andersson C, Narwal R, Janson J, Goldschmidt TJ, Appelkvist P, et al. Sustained peripheral depletion of amyloid-beta with a novel form of neprilysin does not affect central levels of amyloid-beta. Brain. 2014;137(Pt 2):553–64. doi:10.1093/brain/awt308.
Walker JR, Pacoma R, Watson J, Ou W, Alves J, Mason DE, et al. Enhanced proteolytic clearance of plasma Abeta by peripherally administered neprilysin does not result in reduced levels of brain Abeta in mice. J Neurosci. 2013;33(6):2457–64. doi:10.1523/JNEUROSCI.3407-12.2013.
Song ES, Juliano MA, Juliano L, Hersh LB. Substrate activation of insulin-degrading enzyme (insulysin). A potential target for drug development. J Biol Chem. 2003;278(50):49789–94. doi:10.1074/jbc.M308983200.
Abdul-Hay SO, Lane AL, Caulfield TR, Claussin C, Bertrand J, Masson A, et al. Optimization of peptide hydroxamate inhibitors of insulin-degrading enzyme reveals marked substrate-selectivity. J Med Chem. 2013;56(6):2246–55. doi:10.1021/jm301280p.
Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci. 2003;23(6):1992–6.
Spencer B, Marr RA, Rockenstein E, Crews L, Adame A, Potkar R, et al. Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice. BMC Neurosci. 2008;9:109. doi:10.1186/1471-2202-9-109.
Tuszynski MH, Thal L, Pay M, Salmon DP, U H-S, Bakay R et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11(5):551–5. doi:10.1038/nm1239.
Tuszynski MH, Yang JH, Barba D, U H-S, Bakay RA, Pay MM et al. Nerve growth factor gene therapy: activation of neuronal responses in Alzheimer disease. JAMA Neurol. 2015;72(10):1139–47. doi:10.1001/jamaneurol.2015.1807.
Li Y, Wang J, Zhang S, Liu Z. Neprilysin gene transfer: a promising therapeutic approach for Alzheimer’s disease. J Neurosci Res. 2015;93(9):1325–9. doi:10.1002/jnr.23564.
Barua NU, Miners JS, Bienemann AS, Wyatt MJ, Welser K, Tabor AB, et al. Convection-enhanced delivery of neprilysin: a novel amyloid-beta-degrading therapeutic strategy. J Alzheimers Dis. 2012;32(1):43–56. doi:10.3233/JAD-2012-120658.
Iwata N, Sekiguchi M, Hattori Y, Takahashi A, Asai M, Ji B, et al. Global brain delivery of neprilysin gene by intravascular administration of AAV vector in mice. Sci Rep. 2013;3:1472. doi:10.1038/srep01472.
Spencer B, Marr RA, Gindi R, Potkar R, Michael S, Adame A, et al. Peripheral delivery of a CNS targeted, metalo-protease reduces aβ toxicity in a mouse model of Alzheimer’s disease. PLoS One. 2011;6(1):e16575. doi:10.1371/journal.pone.0016575.
Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci USA. 2007;104(18):7594–9. doi:10.1073/pnas.0702170104.
Spencer B, Verma I, Desplats P, Morvinski D, Rockenstein E, Adame A, et al. A neuroprotective brain-penetrating endopeptidase fusion protein ameliorates Alzheimer disease pathology and restores neurogenesis. J Biol Chem. 2014;289(25):17917–31. doi:10.1074/jbc.M114.557439.
Jacobsen JS, Comery TA, Martone RL, Elokdah H, Crandall DL, Oganesian A, et al. Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade. Proc Natl Acad Sci USA. 2008;105(25):8754–9.
Van Nostrand WE, Melchor J, Wagner M, Davis J. Cerebrovascular smooth muscle cell surface fibrillar A beta. Alteration of the proteolytic environment in the cerebral vessel wall. Ann N Y Acad Sci. 2000;903:89–96.
Efanov AM, Barrett DG, Brenner MB, Briggs SL, Delaunois A, Durbin JD, et al. A novel glucokinase activator modulates pancreatic islet and hepatocyte function. Endocrinology. 2005;146(9):3696–701.
Cabrol C, Huzarska MA, Dinolfo C, Rodriguez MC, Reinstatler L, Ni J, et al. Small-molecule activators of insulin-degrading enzyme discovered through high-throughput compound screening. PLoS One. 2009;4(4):e5274.
Bae SJ, Matsunaga Y, Takenaka M, Tanaka Y, Hamazaki Y, Shimizu K, et al. Substance P induced preprotachykinin-a mRNA, neutral endopeptidase mRNA and substance P in cultured normal fibroblasts. Int Arch Allergy Immunol. 2002;127(4):316–21.
Mohajeri MH, Kuehnle K, Li H, Poirier R, Tracy J, Nitsch RM. Anti-amyloid activity of neprilysin in plaque-bearing mouse models of Alzheimer’s disease. FEBS Lett. 2004;562(1–3):16–21.
Mohajeri MH, Wollmer MA, Nitsch RM. Abeta 42-induced increase in neprilysin is associated with prevention of amyloid plaque formation in vivo. J Biol Chem. 2002;277(38):35460–5.
Belyaev ND, Kellett KA, Beckett C, Makova NZ, Revett TJ, Nalivaeva NN, et al. The transcriptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a β-secretase-dependent pathway. J Biol Chem. 2010;285(53):41443–54. doi:10.1074/jbc.M110.141390.
Pardossi-Piquard R, Petit A, Kawarai T, Sunyach C, Alves da Costa C, Vincent B, et al. Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron. 2005;46(4):541–54.
Huang J, Guan H, Booze RM, Eckman CB, Hersh LB. Estrogen regulates neprilysin activity in rat brain. Neurosci Lett. 2004;367(1):85–7. doi:10.1016/j.neulet.2004.05.085.
Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120(5):701–13.
Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, et al. Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat Med. 2005;11(4):434–9. doi:10.1038/nm1206.
Melzig MF, Janka M. Enhancement of neutral endopeptidase activity in SK-N-SH cells by green tea extract. Phytomedicine. 2003;10(6–7):494–8.
Parachikova A, Green KN, Hendrix C, LaFerla FM. Formulation of a medical food cocktail for Alzheimer’s disease: beneficial effects on cognition and neuropathology in a mouse model of the disease. PLoS One. 2010;5(11):e14015. doi:10.1371/journal.pone.0014015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Supported by Grant No. 7-11-CD-06 from the American Diabetes Association to M. A. L. No funds were received specifically for the publication of this review.
Conflicts of interest
The author declares no competing interests.
Rights and permissions
About this article
Cite this article
Leissring, M.A. Aβ-Degrading Proteases: Therapeutic Potential in Alzheimer Disease. CNS Drugs 30, 667–675 (2016). https://doi.org/10.1007/s40263-016-0364-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-016-0364-1