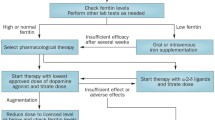
Abstract
An oral, fixed-dose combination of prolonged-release (PR) oxycodone with PR naloxone (Targin®, Targiniq®, Targinact®; hereafter referred to as oxycodone/naloxone PR) is approved in Europe for the second-line symptomatic treatment of patients with severe to very severe idiopathic restless legs syndrome (RLS), after failure of dopaminergic therapy. Coadministration of naloxone represents a targeted approach to counteracting opioid-induced bowel dysfunction without compromising therapeutic efficacy; because of its very low oral bioavailability, naloxone blocks the action of oxycodone at opioid receptors locally in the gut. The efficacy of oxycodone/naloxone PR in patients with severe RLS inadequately controlled by previous (mainly dopaminergic) treatment has been demonstrated in RELOXYN, a 12-week, randomized, double-blind study with a 40-week open-label extension. In this pivotal study, oxycodone/naloxone PR significantly improved RLS symptoms compared with placebo from week 2 onwards; a beneficial effect of oxycodone/naloxone PR was maintained through 1 year of treatment. Furthermore, improvements in RLS symptoms in oxycodone/naloxone PR recipients were accompanied by similarly sustained improvements in disease-specific quality of life and subjective sleep variables. Oxycodone/naloxone PR was generally well tolerated, with a treatment-related adverse event profile (e.g. gastrointestinal disorders, CNS disorders, fatigue and pruritus) that was consistent with that expected for opioid therapy. Notably, there were no confirmed cases of augmentation among oxycodone/naloxone PR recipients throughout the course of the study. Results from the well-designed RELOXYN trial have thus demonstrated the value of oxycodone/naloxone PR as a second-line therapy for severe refractory RLS; further investigation of this combination product as a first-line treatment for severe RLS is now warranted.

Similar content being viewed by others
References
International Restless Legs Syndrome Study Group. 2012 Revised IRLSSG diagnostic criteria for RLS. http://irlssg.org. Accessed 27 Jan 2015.
Kalloo A, Gamaldo CE, Kwan AB, et al. The impact of restless legs syndrome/Willis–Ekbom disorder on quality of life. Eur Neurol Rev. 2013;8(2):97–104.
Salas RE, Kwan AB. The real burden of restless legs syndrome: clinical and economic outcomes. Am J Manag Care. 2012;18:S207–12.
Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012;16(4):987–1007.
Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16(4):283–95.
Jones R, Cavanna AE. The neurobiology and treatment of restless legs syndrome. Behav Neurol. 2013;26(4):283–92.
Ondo WG. Restless legs syndrome: pathophysiology and treatment. Curr Treat Options Neurol. 2014;16(11):317. doi:10.1007/s11940-014-0317-2.
Hornyak M, Scholz H, Kohnen R, et al. What treatment works best for restless legs syndrome? Meta-analyses of dopaminergic and non-dopaminergic medications. Sleep Med Rev. 2014;18(2):153–64.
Klingelhoefer L, Cova I, Gupta S, et al. A review of current treatment strategies for restless legs syndrome (Willis-Ekbom disease). Clin Med. 2014;14(5):520–4.
Rios Romenets S, Postuma RB. Treatment of restless legs syndrome. Curr Treat Options Neurol. 2013;15(4):396–409.
Buchfuhrer MJ. Strategies for the treatment of restless legs syndrome. Neurotherapeutics. 2012;9(4):776–90.
Comella CL. Treatment of restless legs syndrome. Neurotherapeutics. 2014;11:177–87.
Garcia-Borreguero D, Kohnen R, Silber MH. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675–84.
Garcia-Borreguero D, Ferini-Strambi L, Kohnen R, et al. European guidelines on management of restless legs syndrome: report of a joint task force by the European Federation of Neurological Societies, the European Neurological Society and the European Sleep Research Society. Eur J Neurol. 2012;19:1385–96.
Nagandla K, De S. Restless legs syndrome: pathophysiology and modern management. Postgrad Med J. 2013;89:402–10.
Aurora RN, Kristo DA, Bista SR. The treatment of restless legs syndrome and periodic limb movement disorder in adults—an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses. Sleep. 2012;35(8):1039–62.
Garcia-Borreguero D, Stillman P, Benes H. Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol. 2011;11:28. doi:10.1186/471-2377-11-28.
Mundipharma International Ltd. Mundipharma receives positive European Commission decision on Targin® (oxycodone/naloxone) for the treatment of restless legs syndrome [press release]. 2015. http://www.mundipharma.com. Accessed 17 Mar 2015.
European Medicines Agency. Oxynal-Targin and associated names. International non-proprietary name/active substance: oxycodone hydrochloride/naloxone hydrochloride. European public assessment report. 2014. http://www.ema.europa.eu. Accessed 17 Mar 2015.
DePriest AZ, Miller K. Oxycodone/naloxone: role in chronic pain management, opioid-induced constipation, and abuse deterrence. Pain Ther. 2014;3:1–15.
Ahmedzai SH, Leppert W, Janecki M, et al. Long-term safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate-to-severe chronic cancer pain. Support Care Cancer. 2015;23:823–30.
Burness CB, Keating GM. Oxycodone/Naloxone prolonged-release: a review of its use in the management of chronic pain while counteracting opioid-induced constipation. Drugs. 2014;74(3):353–75.
Mundipharma Pharmaceuticals Ltd. Targin® 5 mg/2.5 mg, 10 mg/5 mg, 20 mg/10 mg and 40 mg/20 mg prolonged release tablets. Irish summary of product characteristics. http://www.medicines.ie. Accessed 29 Apr 2015.
Trenkwalder C, Benes H, Grote L, et al. Prolonged release oxycodone-naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurol. 2013;12(12):1141–50.
Kohnen R, Benes H, Grote L, et al. Oxycodone/naloxone prolonged-release has positive quality of life outcomes in patients with severe RLS—results of a 1-year study [abstract no. 2183]. J Neurol Sci. 2013;333:e86.
Benes H, Hornyak M, Hopp M, et al. Prolonged-release oxycodone/naloxone improves sleep quantity and adequacy over 1 year in patients with severe restless legs syndrome [abstract no. PF135]. 15th World Congress on Pain, Buenos Aires; 6–11 Oct 2014.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit. James Frampton is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: A.E. Cavanna, Department of Neuropsychiatry, BSMHFT and University of Birmingham, Birmingham, UK; S. di Biase, Neurology Unit, Department of Experimental and Clinical Medical Sciences, University of Udine Medical School, Udine, Italy; L. Ferini-Strambi, Department of Neurology, Universita Vita-Salute San Raffaele, Milan, Italy; Y. Inoue, Department of Somnology, Tokyo Medical University, Tokyo, Japan.
Rights and permissions
About this article
Cite this article
Frampton, J.E. Oxycodone/Naloxone PR: A Review in Severe Refractory Restless Legs Syndrome. CNS Drugs 29, 511–518 (2015). https://doi.org/10.1007/s40263-015-0254-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-015-0254-y