Abstract
Introduction
Pharmacokinetic outcomes of transporter-mediated drug–drug interactions (TMDDIs) are increasingly being evaluated clinically. The goal of our study was to determine the effects of selective inhibition of multidrug and toxin extrusion protein 1 (MATE1), using famotidine, on the pharmacokinetics and pharmacodynamics of metformin in healthy volunteers.
Methods
Volunteers received metformin alone or with famotidine in a crossover design. As a positive control, the longitudinal effects of famotidine on the plasma levels of creatinine (an endogenous substrate of MATE1) were quantified in parallel. Famotidine unbound concentrations in plasma reached 1 µM, thus exceeding the in vitro concentrations that inhibit MATE1 [concentration of drug producing 50 % inhibition (IC50) 0.25 µM]. Based on current regulatory guidance, these concentrations are expected to inhibit MATE1 clinically [i.e. maximum unbound plasma drug concentration (C max,u)/IC50 >0.1].
Results
Consistent with MATE1 inhibition, famotidine administration significantly altered creatinine plasma and urine levels in opposing directions (p < 0.005). Interestingly, famotidine increased the estimated bioavailability of metformin [cumulative amount of unchanged drug excreted in urine from time zero to infinity (A e∞)/dose; p < 0.005] without affecting its systemic exposure [area under the plasma concentration–time curve (AUC) or maximum concentration in plasma (C max)] as a result of a counteracting increase in metformin renal clearance. Moreover, metformin–famotidine co-therapy caused a transient effect on oral glucose tolerance tests [area under the glucose plasma concentration–time curve between time zero and 0.5 h (AUCglu,0.5); p < 0.005].
Conclusions
These results suggest that famotidine may improve the bioavailability and enhance the renal clearance of metformin.




Similar content being viewed by others
References
Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, et al. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:52–63.
Lu M, Symersky J, Radchenko M, Koide A, Guo Y, Nie R, et al. Structures of a Na+-coupled, substrate-bound MATE multidrug transporter. Proc Natl Acad Sci. 2013;110:2099–104.
Lu M, Radchenko M, Symersky J, Nie R, Guo Y. Structural insights into H+-coupled multidrug extrusion by a MATE transporter. Nat Struct Mol Biol. 2013;20:1310–7.
Damme K, Nies AT, Schaeffeler E, Schwab M. Mammalian MATE (SLC47A) transport proteins: impact on efflux of endogenous substrates and xenobiotics. Drug Metab Rev. 2011;43:499–523.
Motohashi H, Inui K. Multidrug and toxin extrusion family SLC47: physiological, pharmacokinetic and toxicokinetic importance of MATE1 and MATE2-K. Mol Aspects Med. 2013;34:661–8.
Yonezawa A, Inui K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br J Pharmacol. 2011;164:1817–25.
Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem Pharmacol. 2007;74:359–71.
Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006;17:2127–35.
Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci. 2005;102:1–6.
Lee JH, Lee JE, Kim Y, Lee H, Jun H, Lee S. Multidrug and toxic compound extrusion protein-1 (MATE1/SLC47A1) is a novel flavonoid transporter. J Agric Food Chem. 2014;62:9690–8.
Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–90.
Lepist E-I, Zhang X, Hao J, Huang J, Kosaka A, Birkus G, et al. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int. 2014;86:350–7.
Imamura Y, Murayama N, Okudaira N, Kurihara A, Okazaki O, Izumi T, et al. Prediction of fluoroquinolone-induced elevation in serum creatinine levels: a case of drug–endogenous substance interaction involving the inhibition of renal secretion. Clin Pharmacol Ther. 2009;89:81–8.
Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways. Pharmacogenet Genomics. 2012;22:820–7.
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98.
Tsuda M, Terada T, Mizuno T, Katsura T, Shimakura J, Inui K. Targeted disruption of the multidrug and toxin extrusion 1 (mate1) gene in mice reduces renal secretion of metformin. Mol Pharmacol. 2009;75:1280–6.
Toyama K, Yonezawa A, Masuda S, Osawa R, Hosokawa M, Fujimoto S, et al. Loss of multidrug and toxin extrusion 1 (MATE1) is associated with metformin-induced lactic acidosis. Br J Pharmacol. 2012;166:1183–91.
Becker ML, Visser LE, van Schaik RHN, Hofman A. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 2009;58:745–9.
Tkáč I, Klimčáková L, Javorský M, Fabianová M, Schroner Z, Hermanová H, et al. Pharmacogenomic association between a variant in SLC47A1 gene and therapeutic response to metformin in type 2 diabetes. Diabetes Obes Metab. 2013;15:189–91.
Becker ML, Visser LE, van Schaik RHN, Hofman A, Uitterlinden AG, Stricker BHC. Interaction between polymorphisms in the OCT1 and MATE1 transporter and metformin response. Pharmacogenet Genomics. 2010;20:38–44.
Jablonski KA, McAteer JB, de Bakker PIW, Franks PW, Pollin TI, Hanson RL, et al. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the Diabetes Prevention Program. Diabetes. 2010;59:2672–81.
Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A, et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2012;93:186–94.
Kusuhara H, Ito S, Kumagai Y, Jiang M, Shiroshita T, Moriyama Y, et al. Effects of a MATE protein inhibitor, pyrimethamine, on the renal elimination of metformin at oral microdose and at therapeutic dose in healthy subjects. Clin Pharmacol Ther. 2011;89:837–44.
Ito S, Kusuhara H, Kuroiwa Y, Wu C, Moriyama Y, Inoue K, et al. Potent and specific inhibition of mMate1-mediated efflux of type i organic cations in the liver and kidney by pyrimethamine. J Pharmacol Exp Ther. 2010;333:341–50.
Somogyi A, Stockley C, Keal J, Rolan P, Bochner F. Reduction of metformin renal tubular secretion by cimetidine in man. Br J Clin Pharmacol. 1987;23:545–51.
Ha Choi J, Wah Yee S, Kim MJ, Nguyen L, Ho Lee J, Kang J-O, et al. Identification and characterization of novel polymorphisms in the basal promoter of the human transporter, MATE1. Pharmacogenet Genomics. 2009;19:770–80.
Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, et al. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013;56:781–95.
Pepcid label. Merck & Co., Inc.; 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019462s037lbl.pdf.
Yeh KC, Chremos AN, Lin JH, Constanzer ML, Kanovsky SM, Hucker HB, et al. Single-dose pharmacokinetics and bioavailability of famotidine in man. Results of multicenter collaborative studies. Biopharm Drug Dispos. 1987;8:549–60.
Dowling TC, Frye RF, Fraley DS, Matzke GR. Characterization of tubular functional capacity in humans using para-aminohippurate and famotidine. Kidney Int. 2001;59:295–303.
Chremos AN. Clinical pharmacology of famotidine: a summary. J Clin Gastroenterol. 1987;9(Suppl 2):7–12.
Kroemer H, Klotz U. Pharmacokinetics of famotidine in man. Int J Clin Pharmacol Ther Toxicol. 1987;25:458–63.
Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2007;83:273–80.
Lin JH, Los LE, Ulm EH, Duggan DE. Kinetic studies on the competition between famotidine and cimetidine in rats. Evidence of multiple renal secretory systems for organic cations. Drug Metab Dispos. 1988;16:52–6.
Dowling T, Frye R, Fraley D, Matzke G. Characterization of tubular functional capacity in humans using para-aminohippurate and famotidine. Kidney Int. 2001;59:295–303.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Goswami S, Yee SW, Stocker S, Mosley JD, Kubo M, Castro R, et al. Genetic variants in transcription factors are associated with the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2014;96:370–9.
Pentikäinen PJ, Neuvonen PJ, Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16:195–202.
Sambol NC, Chiang J, O’Conner M, Liu CY, Lin ET, Goodman AM, et al. Pharmacokinetics and Pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996;36:1012–21.
Wang Z-J, Yin OQP, Tomlinson B, Chow MSS. OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics. 2008;18:637–45.
Stepensky D, Friedman M, Srour W, Raz I, Hoffman A. Preclinical evaluation of pharmacokinetic–pharmacodynamic rationale for oral CR metformin formulation. J Control Release. 2001;71:107–15.
Zamek-Gliszczynski MJ, Bao JQ, Day JS, Higgins JW. Metformin sinusoidal efflux from the liver is consistent with negligible biliary excretion and absence of enterohepatic cycling. Drug Metab Dispos. 2013;41:1967–71.
Han TK, Proctor WR, Costales CL, Cai H, Everett RS, Thakker DR. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayers. J Pharmacol Exp Ther. 2015;352:519–28.
Nakamichi N, Shima H, Asano S, Ishimoto T, Sugiura T, Matsubara K, et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gastrointestinal absorption of metformin. J Pharm Sci. 2013;102:3407–17.
Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35:1956–62.
Hume WE, Shingaki T, Takashima T, Hashizume Y, Okauchi T, Katayama Y, et al. The synthesis and biodistribution of [(11)C]metformin as a PET probe to study hepatobiliary transport mediated by the multi-drug and toxin extrusion transporter 1 (MATE1) in vivo. Bioorg Med Chem. 2013;21:7584–90.
Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang S-M, et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol. 2013;273:100–9.
Somogyi A, McLean A, Heinzow B. Cimetidine-procainamide pharmacokinetic interaction in man: Evidence of competition for tubular secretion of basic drugs. Eur J Clin Pharmacol. 1983;25:339–45.
Van Crugten J, Bochner F, Keal J, Somogyi A. Selectivity of the cimetidine-induced alterations in the renal handling of organic substrates in humans. Studies with anionic, cationic and zwitterionic drugs. J Pharmacol Exp Ther. 1986;236:481–7.
Yasui-Furukori N, Uno T, Sugawara K, Tateishi T. Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. Clin Pharmacol Ther. 2005;77:17–23.
Ray AS. Creatinine and renal transporters. Endogenous biomarkers: mother nature’s guide to predicting drug–drug interactions. creatine and renal transporters. AAPS annual meeting and exposition; 2–6 Nov 2014: San Diego (CA).
Wang D-S, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510–5.
Motohashi H, Uwai Y, Hiramoto K, Okuda M, Inui K-I. Different transport properties between famotidine and cimetidine by human renal organic ion transporters (SLC22A). Eur J Pharmacol. 2004;503:25–30.
Xia L, Engel K, Zhou M, Wang J. Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Renal Physiol. 2007;292:F682–90.
Acknowledgments
The authors would like to thank the SOPHIE Cohort and Study # 6112 participants for contributing their time. The authors also thank Hector Vizoso, Nurse Manager, and the staff of the CRC, Chav Doherty, Research Study Coordinator, and the staff of the Clinical Laboratory at SFGH for their excellent service and assistance with our clinical study, as well as Dr. Yong Huang, Dr. Howard Horng, and Mr. Nick Massenkoff at the UCSF Drugs Services Unit for access to their bioanalytical facilities and for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institutes of Health (NIH) Grant GM61390, and also by the NIH/National Center for Research Resources (NCRR), University of California, San Francisco (UCSF)—Clinical & Translational Science Institute (CTSI) Grant UL1 RR024131. Jennifer E. Hibma was funded by the National Research Service Award T32 GM07546 from the NIH, and by the Department of Clinical Pharmacy at UCSF. Matthias B. Wittwer was funded by the Swiss National Science Foundation’s grant for prospective researchers (PBBSP3-133384). The authors are solely responsible for the content and do not necessarily represent the official views of the NIH.
Conflict of interest
Kathleen M. Giacomini, Sook Wah Yee, Xuexiang Zhang, and Yong Huang have declared the following conflicts of interest that might be relevant to the content of this manuscript: Kathleen M. Giacomini and Sook Wah Yee are co-founders of Apricity Therapeutics, which develops drugs that exploit membrane transporters to enhance their pharmacologic action. Kathleen M. Giacomini receives funds from several pharmaceutical companies (AstraZeneca, Pfizer, Sanofi-Aventis, and GlaxoSmithKline) for research in her laboratory. Xuexiang Zhang and Yong Huang are employees of Optivia Biotechnology Inc., a transporter contract research organization (CRO) company.
Jennifer E. Hibma, Arik A. Zur, Richard A. Castro, Matthias B. Wittwer, Ron J. Keizer, Srijib Goswami, Sophie L. Stocker, Claire M. Brett, and Radojka M. Savic have no conflicts of interest that might be relevant to the content of this study.
Ethical approval
This analysis was approved by the Committee on Human Research (CHR), which is the Institutional Review Board (IRB) at UCSF, approval # 10-02578.
Additional information
J. E. Hibma and A. A. Zur contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40262_2015_346_MOESM2_ESM.tif
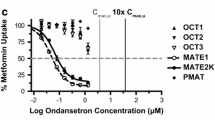
Supplementary material 2: Fig. 1 Famotidine inhibition curves against metformin uptake in HEK293 cell lines stably expressing various transporters; the clinical concentration of famotidine is shaded grey (0-1 µM). Data represent mean ± SEM (n=3). Curves were used to generate half-maximal inhibitory potencies (IC50 values) (TIFF 1521 kb)
40262_2015_346_MOESM3_ESM.tif
Supplementary material 3: Fig. 2 a) Famotidine plasma concentration-time curves from 0-24h following two doses of metformin in healthy volunteers (n=12). Doses of famotidine were administered orally as follows: 200 mg at t=-24h and 160 mg at t=-9, -5, -1, 3 and 7h in relation to the second metformin dose (t=0h). Dashed lines represent the half-maximal inhibitory concentrations (IC50), or multiples of the IC50, for MATE1 and MATE2. b) Ratio of maximal unbound concentration of famotidine in plasma and respective IC50 values, i.e., ([I]/IC50), for organic cation transporters hMATE1 (square), hMATE2 (triangle), hOCT1 (diamond) and hOCT2 (circle). The dashed line represents [I]/IC50 = 0.1. Data represent mean ± SEM (TIFF 1521 kb)
40262_2015_346_MOESM4_ESM.tiff
Supplementary material 4: Fig. 3 Metformin fractional renal clearance (CLR) from 0-2, 2-4, 4-6, 6-8, 8-12, 12-24h after metformin treatment alone (black bars) and during co-administration with famotidine (checkered bars). *P < 0.005. Data represent mean ± SEM (TIFF 20342 kb)
40262_2015_346_MOESM5_ESM.tiff
Supplementary material 5: Fig. 4 Famotidine uptake in cell lines over expressing hOCT1, hOCT2, hMATE1, hMATE2 (black bars) and empty vector (EV) transfected cells (white bars). Famotidine uptake was tested alone (black bars) or together with a specific inhibitor for the relevant transporter (gray bars) (i.e., pyrimethamine (10 µM) for hMATE1/2, cimetidine (500 µM) for hOCT2 and spironolactone (100 µM) for hOCT1). Radioactive counts are normalized per protein amounts in each well and presented as mean ± SEM (n=3) (TIFF 10662 kb)
40262_2015_346_MOESM6_ESM.tiff
Supplementary material 6: Fig. 5 Visual predictive check of final model for population pharmacokinetics of famotidine in plasma in healthy volunteers (n=12). The dashed lines represent the 2.5th percentile, median and 97.5th percentile of the observed data and the shaded area represents the 95% confidence intervals for the 2.5th percentile, median and 97.5th percentile of the simulated data (TIFF 1877 kb)
40262_2015_346_MOESM7_ESM.tiff
Supplementary material 7: Fig. 6 Visual predictive check of final model for population pharmacokinetics of creatinine in plasma in healthy volunteers (n=12). The dashed lines represent the 5th percentile, median and 95th percentile of the observed data and the shaded area represents the 90% confidence intervals for the 5th percentile, median and 95th percentile of the simulated data. (TIFF 1877 kb)
40262_2015_346_MOESM8_ESM.tiff
Supplementary material 8: Fig. 7 Metformin uptake (30uM) in a cell lines transiently expressing hPMAT/ENT4 or mock-transfected cells were compared to uptake in the presence of known ENT4 inhibitor: decynium-22 (100uM) or famotidine (2mM). Data represent mean ± SEM (n=3) (TIFF 6023 kb)
Rights and permissions
About this article
Cite this article
Hibma, J.E., Zur, A.A., Castro, R.A. et al. The Effect of Famotidine, a MATE1-Selective Inhibitor, on the Pharmacokinetics and Pharmacodynamics of Metformin. Clin Pharmacokinet 55, 711–721 (2016). https://doi.org/10.1007/s40262-015-0346-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0346-3